FDA Investigation of Multistate Outbreak of Shiga toxin-producing E. coli Infections Linked to Flour
December 6, 2017
- What was the Problem and What was Done?
- Available Guidance for Industry
- Available Guidance for Consumers
- Additional Resources
What was the Problem and What was Done?
The FDA, CDC, and state and local officials investigated a multi-state outbreak of Shiga toxin-producing Escherichia coli (STEC) O121 and O26 infections.As a result of the investigation, the epidemiologic, traceback, and laboratory information indicated that General Mills flour produced at the Kansas City, Missouri facility was the likely source of this outbreak.
As of September 29, 2016, CDC reported that 63 people infected with the outbreak strains of E. coli O121 and O26 were reported from 24 states. Illnesses started on dates ranging from December 21, 2015 to September 5, 2016. Seventeen ill people were hospitalized, and one person developed hemolytic-uremic syndrome. On September 29, 2016, CDC reported that its outbreak investigation had concluded.
In collaboration with CDC, six cases were initially identified for a traceback investigation. These cases were selected because they: 1) had detailed food histories, 2) had documented purchase information, 3) were geographically distinct, and 4) their clinical isolates were a match by WGS to the outbreak cluster.
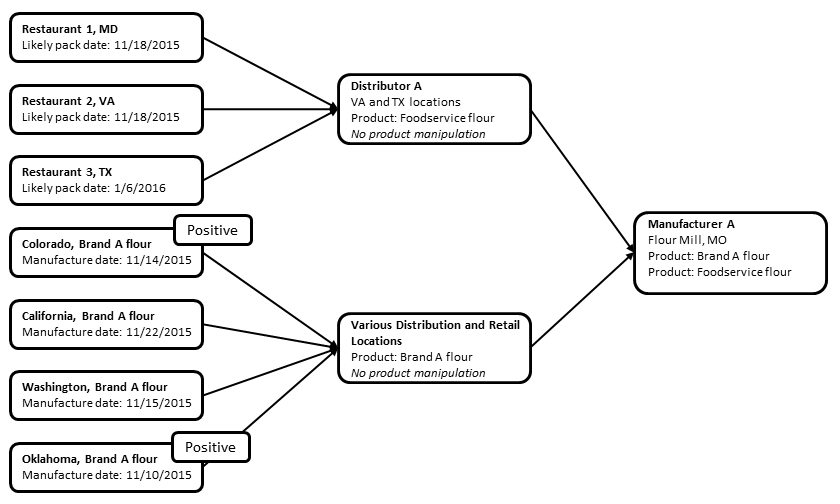
FDA’s initial traceback investigation determined that six of the traceback cases were exposed to General Mills Inc. flour manufactured at a single facility in Kansas City, Missouri. Three cases were exposed to Gold Medal brand flour they had in their homes, and three cases were exposed to raw dough at restaurants. For at least five of these six ill persons, the raw dough eaten or handled was made using General Mills flour that was produced in November 2015.
On May 31, 2016, following a conference call among FDA, CDC, and the firm, General Mills conducted a voluntary recall of flour products produced between November 14, 2015 and December 4, 2015. Recalled products were sold in stores nationwide.
On June 10, 2016, FDA WGS on E. coli O121 isolates recovered from an open sample of General Mills flour, belonging to a Colorado consumer who was sickened, was found to be closely related genetically to the clinical isolates from human illnesses. The flour came from a lot that General Mills had recalled.
Testing by FDA or state laboratories identified E. coli O121 in open product samples collected from ill people in Arizona, Indiana, Michigan, and Oklahoma in addition to Colorado. FDA did WGS analysis of the E.coli O121 from isolates from the Colorado, Michigan, Arizona, and Oklahoma open flour product samples; results show they are closely related genetically to the outbreak strains. The General Mills flour sample collected from the Oklahoma patient was produced outside of the company’s original recall date range and was included in the informational traceback information, diagramed below.
On July 1, 2016, following a call with the FDA and CDC, General Mills expanded its recall of Gold Medal flour, Wondra flour, and Signature Kitchens flour.
General Mills was proactive in their response to this outbreak. They contracted a third-party laboratory to test 50 flour samples not included in the recall for the presence of non-O157 STEC strains. Specifically, the third party lab tested for six STEC strains, including O26, O103, O111, O45, O145 and main outbreak serotype, O121. These serotypes are the most significant illness-causing non-O157 serotypes among STECs. This testing showed that of the 50 samples, five had an STEC strain; one flour sample yielded two strains of STEC. The serotypes of the STECs found in the flour sample were O103, O26, and O111. All of these isolates possessed the virulence characteristics commonly reported in their respective serotypes. Of note, the outbreak strain was not isolated during this testing of additional samples.
The FDA used WGS to characterize isolates provided by General Mills to FDA. FDA provided characterization information to General Mills that an E. coli O26 isolated from their returned retail flour was identified as closely related genetically to a clinical isolate that was subsequently added to the outbreak cluster, based on the WGS information as well as additional epidemiologic information gathered. WGS characterization of the remaining isolates found in General Mills’ flour did not lead to any identification of additional clinical cases related to this outbreak cluster at the time of analysis.
On July 25, 2016, following a call with the FDA and CDC, General Mills expanded its recall a second time to include products produced on select dates through February 10, 2016.
Since the initial recall on May 31, 2016, FDA has facilitated at least 5 recalls of firms that received recalled flour. Listed below are the recalls for which the customer relationship with General Mills has been made public:
| Date | Brand Name | Product Description |
|---|---|---|
| 07/13/2016 | Marie Callender's | Biscuit Mix |
| 07/12/2016 | Golden Dipt | Jalapeno Breader |
| 07/11/2016 | Betty Crocker | Cake Mix |
| 07/09/2016 | Krusteaz | Pancake Mix |
| 08/11/2016 | Rabbit Creek Products | Bread, Muffin & Brownie Mixes |
Available Guidance for Industry:
Some ill people reported handling raw dough at restaurants before eating their meal. Restaurants that allow their customers to handle raw dough should evaluate whether this practice is appropriate.
Restaurants and retailers should be aware that flour may be a source of pathogens and should control the potential for cross-contamination of food processing equipment and the food processing environment. They should follow the steps below:
- Wash and sanitize display cases and refrigerators where potentially contaminated flour was stored.
- Wash and sanitize cutting boards, surfaces, and utensils used to prepare, serve, or store potentially contaminated flour.
- Wash hands with hot water and soap following the cleaning and sanitation process.
- Retailers, restaurants, and other food service operators who have processed and packaged any potentially contaminated products need to be concerned about cross contamination of cutting surfaces and utensils through contact with the potentially contaminated flour.
- Regular frequent cleaning and sanitizing of food contact surfaces and utensils used in food preparation may help to minimize the likelihood of cross-contamination.
See the FDA Bulletin, Food Service and Retail Food Store Industry Regarding Flour and Products Containing Flour, for additional information.
Available Guidance for Consumers:
The recalled General Mills products have a long shelf-life, and may still be present in peoples’ homes. Consumers unaware of the recall could continue to eat these products and potentially get sick.
If consumers have one or more of these products in their homes, they should throw them away. As a precaution, flour no longer stored in its original packaging should be discarded if it could be covered by this recall, and the containers used to store this flour should be thoroughly washed and sanitized.
Three people who became ill reported handling raw dough at restaurants before eating their meal. As a precaution, consumers, especially children, should not handle raw dough at home or at restaurant location; this includes products that do not contain eggs.
FDA warns against eating raw dough products made with any brand of flour or baking mix before cooking. Consumers should always practice safe food handling and preparation measures when handling flour. The FDA recommends following these safe food-handling practices to stay healthy:
- Do not eat or play with any raw cookie dough or any other raw dough or batter product made with flour that is intended to be cooked or baked.
- Follow package directions on baking mixes and other flour-containing products for proper cooking temperatures and for specified times.
- Wash hands, work surfaces, and utensils thoroughly after contact with raw dough products containing flour.
- Keep raw foods separate from other foods while preparing them to prevent any contamination that might be present from spreading.
See the FDA Voice Blog, Sleuthing, and a Little Help from Consumers, Helps FDA Track Down Bacteria in Flour, and Consumer Update, Raw Dough’s a Raw Deal and Could Make You Sick, for additional information.
Additional Resources
FDA Product Isolates
The National Center for Biotechnology Information (NCBI) Pathogen Detection webpage integrates bacterial pathogen genomic sequences originating in food, environmental sources, and patients. It quickly clusters and identifies related sequences to uncover potential food contamination sources, helping public health scientists investigate foodborne disease outbreaks.
During the investigation, WGS data was available for most patients in a cluster of E. coli O121 illnesses. When compared to isolates from open consumer flour samples collected from four different states, the analysis showed that the isolates (clinical and flour) were within 6 SNPs of each other and were considered highly genetically related.
FDA also sequenced six flour isolates provided by General Mills; one of which (FDA00010430) was E. coli O26 and was highly related to a clinical isolate from a patient found to be epidemiologically linked to the outbreak. These two isolates were 3 SNPs away from each other. At the time of investigation, the other five isolates were not found to be closely related to any other isolates in the NCBI database.
The sample numbers for these flour samples are below. The WGS analysis showed that isolates in the main cluster (clinical and flour) were within 6 SNPs of each other and were considered highly genetically related.
FDA flour isolates – main cluster (copy links and paste into your web browser)
Flour isolates provided by General Mills to FDA for WGS analysis (not in main cluster)
FDA00010428
FDA00010429
FDA00010430
FDA00010431
FDA00010432
FDA00010457