Earlier this summer, a burn patient received a minimally invasive skin graft using two BARDA-supported burn care products in tandem, an enzymatic debridement product and a product that promotes skin regeneration. Together these products helped a patient with second degree burns covering much of his body recover quickly – so quickly that he was back to working in his garden after just a few weeks.
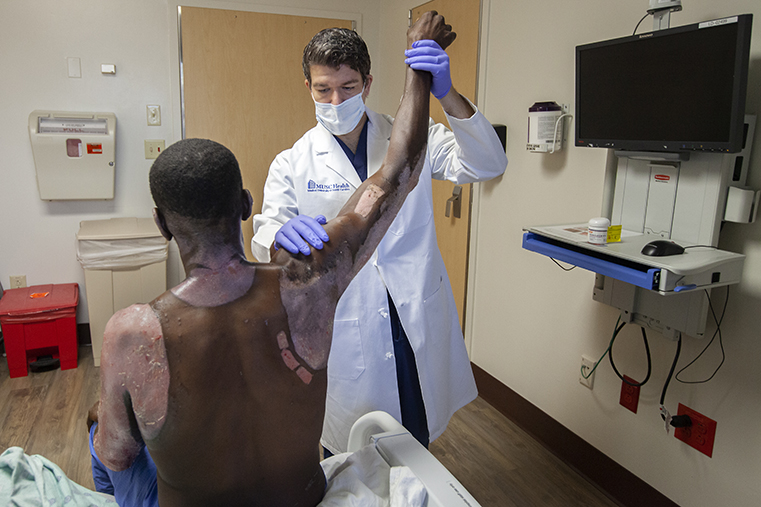
Photo courtesy of the Medical University of South Carolina
The patient was admitted to the burn unit at the Medical University of South Carolina in Charleston. He had sustained second degree burns over 17 percent of his body. Most patients with burns of this magnitude would require surgical debridement of the deep burns and a skin autograft using some of the patient’s remaining skin from another part of the body to cover the wound. Steven Kahn, M.D., and a team of burn care experts at the Medical University of South Carolina took a different approach.
First, Dr. Kahn used an enzymatic treatment that is currently under BARDA-supported clinical development to remove the dead tissue. Then, he used the RECELL system, a product that received FDA-approval in 2018 after receiving support from BARDA, to promote skin regeneration using only a small sample of the patient’s skin. By using both products, he was able to avoid the use of traditional surgical excision debriding and skin graft procedures, both of which can be time consuming and painful, and require the skills of highly trained burn care specialists.
At ASPR/BARDA, we are committed to developing medical countermeasures and technologies that can save lives and promote the recovery of people who are injured or develop an infection during a disaster or emergency. Burn injuries are debilitating and a direct result of many of the potential incidents that threaten our nation’s health security.
Since 2013 BARDA’s collaboration with the American Burn Association has been instrumental in identifying opportunities for improvement across the continuum of burn care. After extensive analysis, BARDA identified two critical bottlenecks that could hamper care during a mass casualty incident (MCI): (1) limited treatment capacity, and (2) inherent inefficiencies in procedures.
Accordingly, BARDA has invested in a portfolio of cutting-edge technologies of medical countermeasures (MCMs) which specifically improve treatment across the burn care continuum ranging from wound assessment, debridement, through skin coverage and regeneration. BARDA’s investments in novel products are directed at specific steps in the progression and treatments in the burn care continuum. In doing so they introduce new ways to be efficient and expand capacity, the bottlenecks in the delivery of burn care in our national context.
Current burn care always requires a highly skilled surgeon to debride and excise the wounds, which is the process of carefully removing the dead tissue. With limitations in the number of surgeons in the country who are trained to perform these procedures that are both time consuming and traumatic for the patient, investing in alternatives is imperative.
BARDA has invested in a novel enzymatic debridement product that is both easier to administer than the current practice and it is incredibly precise. This promising enzymatic debridement product can remove dead tissue without damaging the surrounding live tissue. This level of precision is hard to achieve surgically. If the product receives FDA approval, many believe it has the potential to change the resource and surgical needs and enable clinicians to treat more patients quickly which is especially important during an MCI. BARDA is supporting its advanced development for introduction in the U.S. and is currently under Biologics License Application (BLA) review by the Food and Drug Administration (FDA).
To address the second bottleneck, BARDA is supporting innovative approaches to increase efficiency and reduce the need to harvest a patient’s own skin, a procedure known as autografting. Typically, skin grafting requires a donor site from another part of the patient’s body. The procedure is often quite painful and can result in additional scarring. BARDA’s support to Avita’s RECELL Spray on Skin technology received FDA approval in 2018. Like autografting, this technology requires the removal of a small patch of skin from the patient, which is then converted into a spray solution that can cover a much larger wound surface area than a standard skin graft could.
In the photo above, the three rectangles on the patient’s back are the donor site. Dr. Kahn estimated that the following conventional approach of care would have needed most of the skin from the front of the patient’s thighs. RECELL can be used to produce a skin cell spray that can cover 80 times the area of the donor site. In this case, those three small rectangles of skin were enough to treat the burns that covered 17 percent of his body. By using RECELL Spray on Skin, the burn surgeon was able to help the patient’s wounds heal with a minimally invasive procedure that was less painful and regenerated skin tissue quicker.
Just a couple of weeks after this burn injury, the patient reported that he was feeling great. He explained that the pigmentation of his new skin is even; his skin isn’t tight, itchy or painful. He has gone out and done some yard work - while covering up and keeping out of full sunshine. That is an outstanding patient outcome for a person who had second degree burns covering 17 percent of his body
For BARDA, there are key metrics to measure from the benefits these new MCMs targeted within the continuum of care program. One is whether the MCMs shorten the hospital stays for the patients. Dr. Kahn estimated that he normally would have kept such a patient in the hospital for 19 days. This patient was able to be discharged after only 12 days. This information is important for both BARDA and the community of care providers. For BARDA this data metric reflects a substantial return on investment, strengthening preparedness for a MCI and improving the standard patient care every day.
Dr. Kahn has indicated that the patient’s rapid recovery would not have been possible without the non-surgical enzymatic debridement approach combined with RECELL. By using these two cutting-edge products together and taking an end-to-end approach to burn care, Dr. Kahn was able to provide next-generation burn care to his patient.
While products that address each of these treatment targets can independently improve burn care, use of multiple products could have an even greater impact on patient health when they are used together in tandem. At BARDA, we envision such synergy further increasing the efficiency of burn care when all these new products would ultimately get integrated into routine burn care.
The overall quality of the national burn care system would consequently improve as well leading to speed recovery times, reduced pain and scarring, and improve patient outcomes. In an MCI, technologies that alleviate bottlenecks and enable doctors to treat patients more effectively and efficiently could, in turn, help doctors treat more patients and save more lives.
The successful patient outcome that resulted from the synergistic use of these two products that BARDA has funded demonstrates how we are fulfilling our mission to protect health and save lives through partnership with the private industry. This example helps validate the underlying strategy of integrating overlapping MCMs across the care continuum into routine clinical practice which builds synergy, value, and improves national preparedness over the long term.
Check out the MUSC Catalyst News to learn more about the treatment this patient received from the team at the Medical University of South Carolina.

