Aquatics FAQs
Recommendations for Fully Vaccinated People
COVID-19 Homepage
Optimizing Supply of PPE and Other Equipment during Shortages
Personal protective equipment (PPE) is used every day by healthcare personnel (HCP) to protect themselves, patients, and others when providing care. PPE helps protect HCP from many hazards encountered in healthcare facilities.
The greatly increased need for PPE caused by the COVID-19 pandemic has caused PPE shortages, posing a tremendous challenge to the U.S. healthcare system. Healthcare facilities are having difficulty accessing the needed PPE and are having to identify alternate ways to provide patient care.
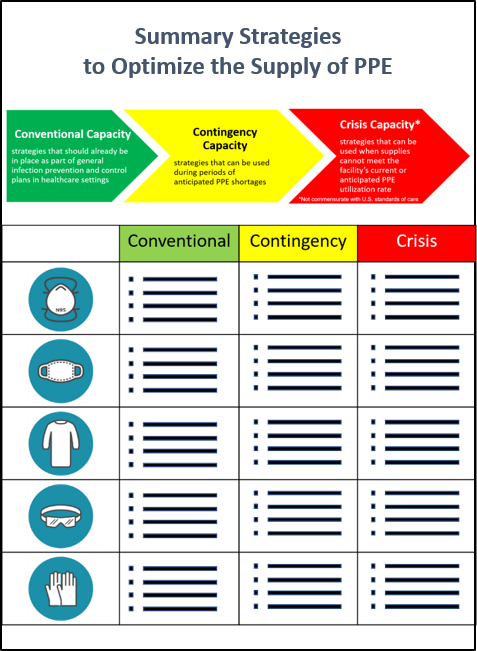
Surge capacity refers to the ability to manage a sudden increase in patient volume that would severely challenge or exceed the present capacity of a facility. While there are no commonly accepted measurements or triggers to distinguish surge capacity from daily patient care capacity, surge capacity is a useful framework to approach a decreased supply of PPE during the COVID-19 response. To help healthcare facilities plan and optimize the use of PPE in response to COVID-19, CDC has developed a Personal Protective Equipment (PPE) Burn Rate Calculator. Three general strata have been used to describe surge capacity and can be used to prioritize measures to conserve PPE supplies along the continuum of care.
- Conventional capacity: measures consisting of engineering, administrative, and PPE controls that should already be implemented in general infection prevention and control plans in healthcare settings.
- Contingency capacity: measures that may be used temporarily during periods of anticipated PPE shortages. Contingency capacity strategies should only be implemented after considering and implementing conventional capacity strategies. While current supply may meet the facility’s current or anticipated utilization rate, there may be uncertainty if future supply will be adequate and, therefore, contingency capacity strategies may be needed.
- Crisis capacity: strategies that are not commensurate with U.S. standards of care but may need to be considered during periods of known PPE shortages. Crisis capacity strategies should only be implemented after considering and implementing conventional and contingency capacity strategies. Facilities can consider crisis capacity strategies when the supply is not able to meet the facility’s current or anticipated utilization rate.
CDC’s optimization strategies for PPE offer a continuum of options for use when PPE supplies are stressed, running low, or exhausted. Contingency and then crisis capacity measures augment conventional capacity measures and are meant to be considered and implemented sequentially. As PPE availability returns to normal, healthcare facilities should promptly resume standard practices.
Decisions to implement contingency and crisis strategies are based on these assumptions:
- Facilities understand their current PPE inventory and supply chain
- Facilities understand their PPE utilization rate
- Facilities are in communication with local healthcare coalitions and federal, state, and local public health partners (e.g., public health emergency preparedness and response staff) to identify additional supplies
- Facilities have already implemented conventional capacity measures
- Facilities have provided HCP with required education and training, including having them demonstrate competency with donning and doffing, with any PPE ensemble that is used to perform job responsibilities, such as provision of patient care
HCP and facilities—along with their healthcare coalitions, local and state health departments, and local and state partners—should work together to develop strategies that identify and extend PPE supplies, so that recommended PPE will be available when needed most. When using PPE optimization strategies, training on PPE use, including proper donning and doffing procedures, must be provided to HCP before they carry out patient care activities.
Emergency Use Authorization (EUA) of Respiratory Protective Devices
On February 4, 2020, the HHS Secretary declared that circumstances exist to justify the authorization of emergency use of additional respiratory protective devices in healthcare settings during the COVID-19 outbreak. The FDA is providing frequent updates for manufacturers, facilities, and local/state jurisdictions about Emergency Use Authorizations (EUA) for respirators and other types of personal protective equipment. The FDA has issued EUAs to authorize all NIOSH approved particulate-filtering air purifying respirators (APRs)external icon to be used in healthcare settings, including all NIOSH approved filtering facepiece respirators, elastomeric APRs, powered air purifying respirators, expired NIOSH-approved filtering facepiece respirators, and respirators that have been decontaminated pursuant to the terms and conditions of an authorized decontamination system. The authorized decontamination systems are listed on the FDA EUA website. In addition, non-NIOSH-approved disposable filtering facepiece respirators within the context of the posted EUAexternal icon are permitted for use as well.