Spotlight on Science - Fall 2020
In This Issue…
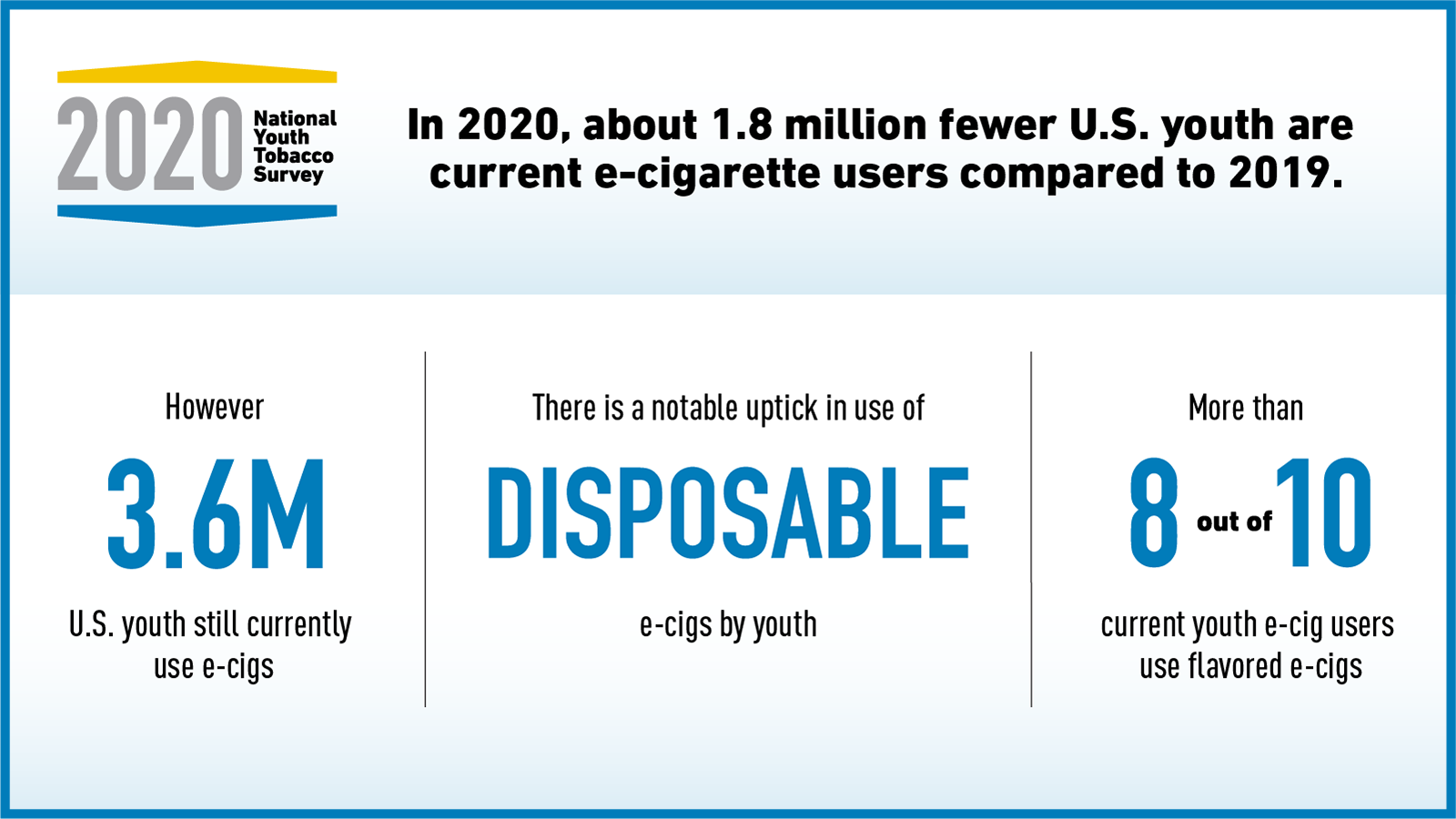
The 2020 National Youth Tobacco Survey (NYTS) shows that 1.8 million fewer youth are using e-cigarettes but 3.6 million youth are still currently using them. Learn more in this latest issue of Spotlight on Science, a quarterly research and science digest from FDA’s Center for Tobacco Products (CTP).
- NYTS Shows Encouraging Decline in ENDS Use But Concerning Uptick in Disposables
- PATH Study Update: Special Collection of Youth Only Public-Use Files
- PATH Restricted-Use Biomarker Data Available for Researchers
- CTP Director’s Perspective on the Sept. 9 Deadline
- FDA Releases Updated Version of the Safety Reporting Portal’s Tobacco Questionnaires
- Tips for Safe Disposal of E-Cigarettes and E-Liquid Waste
- More About CTP Research
NYTS Shows Encouraging Decline in ENDS Use But Concerning Uptick in Disposables
FDA and the Centers for Disease Control and Prevention (CDC) released new data from the 2020 National Youth Tobacco Survey (NYTS), which show 1.8 million fewer U.S. youth are currently using e-cigarettes compared to 2019. After two years of disturbing increases in youth e-cigarette use, we are encouraged by the overall significant decline reported in 2020. This is good news; however, the FDA remains very concerned about the 3.6 million U.S. youth who currently use e-cigarettes. We acknowledge there is work that still needs to be done to curb youth use.
The 2020 NYTS data also show an alarming uptick in use of disposable e-cigarettes by youth. Specifically, disposable e-cigarettes are being used by 26.5% of high school e-cigarette users (up from 2.4% in 2019) and 15.2% of middle school e-cigarette users (up from 3% in 2019). Furthermore, more than 8 out of 10 youth e-cigarette users report use of flavored products, with fruit, mint, candy, and menthol among the most commonly used. This is the first year in which the NYTS distinguished between mint and menthol products; in previous years’ surveys, youth were asked questions in which products were identified as “mint/menthol” and not further delineated.
PATH Study Update: Special Collection of Youth Only Public-Use Files
CTP and the National Institutes of Health (NIH) announce the release of the Special Collection Wave 4.5 Youth Only Public-Use Files. This data release consists of questionnaire data collected only from youth respondents (12-17 years) between Waves 4 and 5 of the Population Assessment of Tobacco and Health (PATH) Study (from December 2017 to November 2018) and are available for download.
The restricted-use version of the file that was made available to researchers upon request was released earlier in 2020. CTP OS researchers can access internal Restricted Use Files (RUFs) for approved projects or submit data analysis requests through the PATH Study mailbox at [email protected]
Additional information about the PATH Study, a household-based, nationally representative, longitudinal cohort study of youth (12-17 years old) and adults in the United States, can be found in the “For Researchers” section of the updated PATH Study Series Page.
PATH Restricted-Use Biomarker Data Available for Researchers
CTP and NIH announce the availability of the restricted-use biomarker data files from the fourth wave of the PATH Study, in addition to updates to restricted-use biomarker data files from Waves 2 and 3 of the study. These latest additions to the PATH Study’s Biomarker Restricted-Use Files (BRUF) contain data collected during Wave 4 (December 2016 – January 2018), Wave 3 (October 2015 – October 2016), and Wave 2 (October 2014 – October 2015). Qualified researchers may apply for access through the PATH Study Biomarker Restricted-Use Files webpage.
The newly released BRUF data files include the following:
Wave 2
- Urine Panel - Oxidative Stress (F2PG2a)
- Urine Biomarker Weights – Oxidative Stress (F2G2a)
Wave 3
- Urine Panel - Hydroxy Polycyclic Aromatic Hydrocarbons
Wave 4
- Urine Collection and Nicotine Exposure Questions Data
- Urine Biomarker Weights - All-Waves Urine Weights
- Urine Biomarker Weights - Single-Wave Urine Weights
- Urine Panel - Enzymatic Urinary Creatinine
- Urine Panel - Metals
- Urine Panel - Urinary Nicotine Metabolites (Cotinine and Hydroxycotinine)
CTP Director’s Perspective on the Sept. 9 Deadline
On Sept. 9, 2020, premarket review applications for many new tobacco products currently on the market, including e-cigarettes, certain cigars, and hookah products, were due to FDA for review. FDA staff have been working tirelessly on preparations. At the end of August, CTP Director Mitch Zeller published a perspective piece on CTP’s preparations to meet this challenge in an efficient, reliable and transparent manner. The perspective piece covers many issues, including expectations for the applications, preparations for receiving and processing the applications efficiently, and how CTP plans to handle review of this large influx of applications.
The Deeming Rule and FDA’s Premarket Review Requirement for Deemed Tobacco Products
By Mitch Zeller, Director of the FDA’s Center for Tobacco Products (CTP), Published August 31, 2020
Prior to Aug. 8, 2016, e-cigarettes, cigars and hookah products were not regulated by FDA. That’s because the original grant of authority from Congress in 2009 only covered cigarettes, smokeless tobacco, cigarette tobacco, and roll-your-own tobacco.
That all changed with FDA’s historic “deeming rule” that helps implement the Tobacco Control Act and allows FDA to protect the public health and protect future generations from the dangers of tobacco use.
On Aug. 8, 2016, when the deeming rule took effect, many of the regulatory and statutory requirements that had been in place for manufacturers of cigarettes, smokeless tobacco, cigarette tobacco, and roll-your-own tobacco since 2009, became applicable to e-cigarettes and all other electronic nicotine delivery systems (ENDS), cigars, pipe tobacco, nicotine gels, hookah tobacco, and any future tobacco products.
FDA Releases Updated Version of the Safety Reporting Portal’s Tobacco Questionnaires
FDA has made several updates to the full Safety Reporting Portal (SRP) questionnaires to better capture information about affected individuals, pre-existing health conditions, and product-specific details. For example:
- All voluntary reports must now include the affected person’s age, as well as how many people were affected, and whether nonusers were affected.
- E-cigarette or vape users are asked how much nicotine is in the tobacco product that seemed to cause the problem.
- Optional questions about health problems have been expanded to assess specifically for physical or mental disabilities and English language ability.
- Optional questions related to e-cigarette or vape products now request more information about the product’s battery, its operating status, and other details to help identify the specific product involved.
For researchers providing voluntary reports of adverse experiences that occurred during a study, information on expectedness and relatedness of the problem to the study tobacco product is now required. Research group accounts can also add multiple e-mail addresses to be notified when a report is submitted.
FDA encourages anyone who has had a reaction to, or was hurt by, a tobacco product – or knows someone who experienced such effects – to visit the SRP and provide as much information as possible. Reports may be submitted anonymously but including contact information allows FDA the option to find out more about the adverse experience. FDA reviews all tobacco-related SRP reports to identify new or concerning trends.
Tips for Safe Disposal of E-Cigarettes and E-Liquid Waste
E-cigarettes, including rechargeable batteries and the cartridges and bottles that contain e-liquids (liquid nicotine mixtures), can pose a threat to human health and to the environment if they are not disposed of properly. E-cigarette and e-liquid waste should not be thrown in the regular trash or flushed down a sink. Instead, these items should be taken safely to a hazardous waste facility.
More About CTP Research
Recent Publications by CTP Researchers
- School use and normative perceptions of electronic nicotine product use among middle and high school students--November 2018. J Adolesc Health 2020 Aug 1 [Epub ahead of print]
- Predicting the mutagenic potential of chemicals in tobacco products using in silico toxicology tools. Toxicol Mech Methods 2020 Aug 4 [Epub ahead of print]
- Urinary biomarkers of exposure to volatile organic compounds from the Population Assessment of Tobacco and Health Study Wave 1 (2013-2014). Int J Environ Res Public Health 2020 Jul 28;17(15):E5408
- Nicotine exposure by device type among adult electronic nicotine delivery system users in the Population Assessment of Tobacco and Health study, 2015-2016. Cancer Epidemiol Biomarkers Prev 2020 Jul 29 [Epub ahead of print]
- Tobacco-specific nitrosamines (NNAL, NNN, NAT, and NAB) exposures in the US Population Assessment of Tobacco and Health (PATH) Study Wave 1 (2013-2014). Nicotine Tob Res 2020 Jul 27 [Epub ahead of print]
- Burn injuries related to e-cigarettes reported to poison control centers in the United States, 2010-2019. Inj Epidemiol 2020 Jul 20;7(1):36
- Cigalike electronic nicotine delivery systems e-liquids contain variable levels of metals. Sci Rep 2020 Jul 17;10(1):11907
- In vitro and in silico genetic toxicity screening of flavor compounds and other ingredients in tobacco products with emphasis on ENDS. J Appl Toxicol 2020 Jul 13 [Epub ahead of print]
- Predictive validity of the adult tobacco dependence index: Findings from waves 1 and 2 of the Population Assessment of Tobacco and Health (PATH) study. Drug Alcohol Depend 2020 Sep 1;214:108134
Research Opportunities to Explore
In support of its mission to develop regulation rooted in science, CTP seeks new research to address key areas of tobacco regulatory science such as:
- Toxicity
- Addiction
- Health effects
- Behavior
- Communications
- Marketing influences
- Impact analysis of regulatory actions
CTP encourages research studies to include, where appropriate to the research question, populations of special relevance, including (but not limited to): youth, socioeconomically disadvantaged populations, racial/ethnic minorities, underserved rural populations, people with co-morbid mental health conditions and/or substance use disorders, military/veteran populations, pregnant women or women of reproductive age, and sexual and gender minorities.
Featured below are some current CTP funding opportunities. You can find more information by clicking on the appropriate funding opportunity announcement (FOA) number.
More information on CTP-funded research can be found on the center’s website.
NIH Tobacco Regulatory Science Program
The Tobacco Regulatory Science Program, FDA’s partnership with NIH to foster tobacco regulatory research, offers funding for scientists whose research can inform CTP’s regulatory activities.
Tobacco Regulatory Science (R01 Clinical Trial Optional)
Application due date: February 13, 2021, by 5:00 PM local time of applicant organization.
The purpose of this Funding Opportunity Announcement (FOA) is to invite R01 applications to support biomedical and behavioral research that will provide scientific data to inform regulation of tobacco products to protect public health. Research Projects must address the research priorities related to the regulatory authority of the Food and Drug Administration (FDA) Center for Tobacco Products (CTP). The awards under this FOA will be administered by NIH using funds that have been made available through FDA CTP and the Family Smoking Prevention and Tobacco Control Act (P.L. 111-31). Research results from this FOA are expected to generate findings and data that are directly relevant in informing the FDA's regulation of the manufacture, distribution, and marketing of tobacco products to protect public health.
Notice of Special Interest: Research to Advance the Understanding of Tobacco Product Pharmacokinetic Research (NOT-OD-20-081)
Application due date: February 13, 2021, by 5:00 PM local time of applicant organization.
The purpose of this Notice is to invite R01 applications focused on Tobacco Product Pharmacokinetic Research to support biomedical and behavioral research that will provide scientific data to inform the regulation of tobacco products to protect public health.
Maximizing the Scientific Value of Existing Biospecimen Collections: Scientific Opportunities for Exploratory Research (R21 Clinical Trial Not Allowed) (RFA-OD-19-021)
Application due dates: March 8, 2021, by 5:00 PM local time of applicant organization.
This Funding Opportunity Announcement (FOA) invites R21 applications to stimulate exploratory research relevant to the mission of the FDA Center for Tobacco Products (CTP) using existing (publicly available) biospecimens currently stored in repositories in the United States. This will include, but not be limited to, collections associated with the Population Assessment of Tobacco and Health (PATH) Study, the National Health and Nutrition Examination Survey (NHANES), the National Heart, Lung and Blood Institute’s (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC), and the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Proposed research should seek to maximize the scientific value of these stored collections and to provide researchers with an opportunity to generate preliminary data for subsequent research proposals. While other publicly available repositories would be considered, depending on analyses to be conducted, nationally representative analyses will receive priority. These applications need to provide justification why the data set is unique, and the research questions cannot be answered from a publicly available, nationally representative, data set.
Secondary Analyses of Existing Datasets of Tobacco Use and Health (R21 Clinical Trial Not Allowed) (RFA-OD-19-022)
Application due dates: March 8, 2021, by 5:00 PM local time of applicant organization.
This Funding Opportunity Announcement (FOA) invites R21 applications proposing the innovative analysis of existing (publicly available) nationally representative U.S. cross-sectional and longitudinal data, to investigate novel scientific ideas and/or to generate new models, systems, tools, methods, or technologies that have the potential for significant impact on biomedical or biobehavioral research in areas relevant to the FDA Center for Tobacco Products (CTP). Other publicly available data sets would be considered depending on the analyses to be conducted; however, nationally representative analyses will receive priority. Applications not using nationally representative data sets will need to provide justification why the data set is unique, and why the research questions cannot be answered from a (publicly available) nationally representative data set. This FOA encourages the analyses of public use datasets that may inform tobacco regulatory actions in the United States.
FDA Support for Conferences and Scientific Meetings (R13)
Application due dates: April 12, 2021; October 12, 2021; April 12, 2022; and October 11, 2022 by 11:59 p.m. EST.
FDA recognizes the value of supporting high quality, small scientific conferences. A small scientific conference is defined as a symposium, seminar, workshop, or any formal meeting, whether conducted face-to-face or virtually to exchange information and explore a defined subject, issue, or area of concern that impact the public's health within the scope of FDA's mission.
Mentored Research Scientist Career Development Award in Tobacco Regulatory Research (K01; RFA-OD-20-008 or RFA-OD-20-011)
Application due dates: Feb. 8, 2021, Oct. 8, 2021, and July 8, 2022 by 5:00 p.m. local time of applicant organization.
This award provides support for an intensive, supervised career development experience of three, four, or five protected years in biomedical, behavioral, and social science research. This experience will inform the development and evaluation of tobacco regulation and will foster research independence. Projects must address one of FDA’s research priority areas.
Pathway to Independence Award in Tobacco Regulatory Research (K99/R00; RFA-OD-20-009 or RFA-OD-20-010)
Application due dates: Feb. 8, 2021, Oct. 8, 2021, and July 8, 2022 by 5:00 p.m. local time of applicant organization.
This FOA aims to increase and maintain a strong cohort of talented independent investigators conducting research that will inform the development and evaluation of tobacco product regulations. This program is designed to facilitate the timely transition of outstanding postdoctoral researchers from mentored, postdoctoral research positions to independent, tenure-track or equivalent faculty positions. This program will provide NIH research support during this transition in order to help awardees launch competitive, independent research careers.
Spotlight on Science is a quarterly science and research digest from FDA’s Center for Tobacco Products.
Note: The contents of publications discussed in this newsletter are the responsibility of their authors alone, and findings and conclusions are those of the authors and do not necessarily represent the views of FDA.