Assessing the Respiratory Effects of Approved Opioid Products When Co-administered with Commonly Prescribed Drugs
Ventilation (breathing) is tightly controlled by feedback mechanisms involving carbon dioxide. When the breathing of an individual is restricted or stopped, the exhalation of carbon dioxide is similarly curtailed. As the level of carbon dioxide in the blood rises, chemical receptors in the body signal the lungs to increase ventilation. Opioids decrease this ventilatory response to carbon dioxide, which can result in severe respiratory depression and death. Some drugs, such as benzodiazepines, can further decrease breathing when used in combination with opioids.
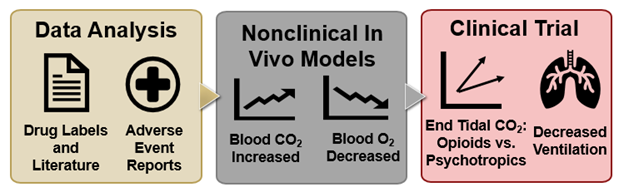
In August 2016, the FDA required safety labeling changes, including the addition of boxed warnings, for benzodiazepine and opioid products, to indicate that their combined use could increase the potential for respiratory depression. Following this action, the FDA undertook a multi-step approach (Figure 1) to assess whether drugs other than benzodiazepines, if similarly co-administered with opioid drugs, might also raise the potential risk for respiratory depression.
Nonclinical Studies to Assess 15 Psychotropic Drugs in Combination with an Opioid
In the wake of the safety labeling changes required for benzodiazepine and opioid products in August 2016, the FDA sought to better understand the potential risk for respiratory depression associated with additional FDA-approved drugs (i.e., other than benzodiazepines) when administered in combination with opioid drugs. Accordingly, the FDA reviewed the published literature and identified 15 psychotropic drugs, which might be prescribed to substitute for diazepines, for further evaluation. Accordingly, CDER researchers investigated the respiratory effects of psychotropic drugs,, 1 when combined with opioid administration, in the in vivo rat model previously described by CDER scientists,2 measuring whether arterial carbon dioxide increased and if arterial oxygen decreased following co-administration of the specified psychotropic and opioid drugs. From those studies, the selective serotonin reuptake inhibitor (SSRI) paroxetine and the atypical antipsychotic quetiapine were found to increase respiratory depression when combined with oxycodone, compared in each case to oxycodone alone. In light of these results, CDER selected paroxetine and quetiapine for further evaluation of respiratory effects, in the context of co-administration with opioid medication, in a clinical trial.3
Clinical Trial Background and Methodology
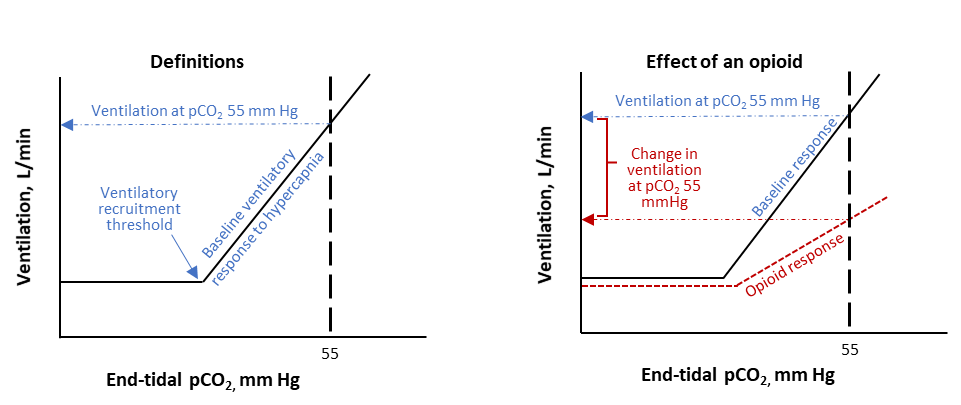
FDA scientists evaluated study designs that would allow a safe and controlled assessment of the effects of drug combinations on respiratory depression. Changes in ventilation were assessed using a breathing procedure where participants rebreathe through a closed circuit with elevated levels of carbon dioxide, resulting in increasing levels of carbon dioxide in the participants’ lungs, blood, and brain. This increase in carbon dioxide stimulates individuals to increase their ventilation (Figure 2). To safely study the effects of paroxetine and quetiapine alone and in combination with an opioid on ventilation in humans, doses of each drug were selected that would not cause substantial effects on ventilation when breathing room air but may have detectable effects under hypercapnic conditions (i.e., when blood levels of carbon dioxide are high). Using this design, FDA assessed how ventilation changed when participants were taking paroxetine plus oxycodone or quetiapine plus oxycodone compared to placebo plus oxycodone in a randomized clinical trial.
Clinical Trial Findings
In a randomized crossover clinical trial, 25 healthy participants received either paroxetine, quetiapine, or placebo over five days. Oxycodone was administered on the first and fifth day of study drug administration so effects on hypercapnic ventilation could be determined after a single dose or with repeat administration of study drugs. The breathing procedure described above was conducted at multiple timepoints pre- and post-dosing to evaluate the effects of the drug combinations on hypercapnic ventilation compared to placebo plus oxycodone. The study also included rebreathing assessments on the fourth day of the study, where no oxycodone was administered, providing a comparison of the effects of paroxetine or quetiapine with placebo on hypercapnic ventilation.
The study showed that paroxetine plus oxycodone significantly decreased mean hypercapnic ventilation compared to placebo plus oxycodone. Quetiapine plus oxycodone, in contrast, did not show any additional effects compared to oxycodone alone. Observations from study days where paroxetine was administered alone indicated that paroxetine had an independent effect on the ventilatory response to hypercapnia compared to placebo. Furthermore, concentration-response modeling showed that the observations from paroxetine plus oxycodone occur outside of the range of effects on ventilation modeled by variation of oxycodone concentration alone.3
Applying Research Findings to Address the Opioid Crisis
The finding that paroxetine combined with oxycodone, compared to oxycodone alone, decreased the hypercapnic ventilatory response is concerning because the hypercapnic ventilatory response is the primary feedback mechanism for the body to rescue itself from opioid-induced respiratory depression. Additionally, the observation that paroxetine decreased ventilation compared to placebo suggests that paroxetine decreased the ventilatory response to hypercapnia through a direct pharmacodynamic effect, rather than a pharmacokinetic interaction between the SSRI and opioid drugs.
This clinical trial is a part of the FDA’s proactive work to address the opioid crisis and help reduce opioid overdoses and deaths, and more specifically demonstrates that drugs that might be used in place of benzodiazepines may also exacerbate opioid-induced respiratory depression. The findings may have important clinical implications for patients on paroxetine (or, potentially, other antidepressant drugs) who concomitantly use opioids, but further research is needed. FDA researchers have initiated a separate clinical trial to further explore the observations with paroxetine and better understand the dosing effects on ventilation during paroxetine treatment and related drugs. In addition, this study serves as a proof-of-concept for how this methodology could be prospectively used for evaluating effects of investigational drugs alone or in combination with other drugs on ventilation.
The finding that paroxetine combined with oxycodone, compared to oxycodone alone, decreased the hypercapnic ventilatory response is concerning because the hypercapnic ventilatory response is the primary feedback mechanism for the body to rescue itself from opioid-induced respiratory depression. Additionally, the observation that paroxetine decreased ventilation compared to placebo suggests that paroxetine decreased the ventilatory response to hypercapnia through a direct pharmacodynamic effect, rather than a pharmacokinetic interaction between the SSRI and opioid drugs. This clinical trial is a part of the FDA’s proactive work to address the opioid crisis and help reduce opioid overdoses and deaths, and more specifically demonstrates that drugs that might be used in place of benzodiazepines may also exacerbate opioid-induced respiratory depression. The findings may have important clinical implications for patients on paroxetine (or, potentially, other antidepressant drugs) who concomitantly use opioids, but further research is needed. FDA researchers have initiated a separate clinical trial to further explore the observations with paroxetine and better understand the dosing effects on ventilation during paroxetine treatment and related drugs. In addition, this study serves as a proof-of-concept for how this methodology could be prospectively used for evaluating effects of investigational drugs alone or in combination with other drugs on ventilation.
Figure 1. Opioid Drug-Drug Interaction Effects on Respiratory Depression. Supported by data from the literature and adverse event reports, FDA conducted a nonclinical in vivo study to assess effects on ventilation for drugs given alone or in combination with an opioid. Subsequently, an FDA-led clinical trial assessed the effects of those drugs (benzodiazepines and others) in healthy participants receiving an opioid.
Figure 2. Illustration of the Ventilatory Response to Hypercapnia and How Opioids Can Affect the Response. Ventilation increases at an approximately linear rate after carbon dioxide (pCO2) is higher than the ventilatory recruitment threshold (VRT). Opioids cause small decreases in ventilation below the VRT, shift the VRT to the right, and decrease the rate of rise in ventilation as pCO2 increases further.
1Xu L, Krishna A, Stewart S, Shea K, Racz R, Weaver JL, Volpe DA, Pilli NR, Narayanasamy S, Florian J, Patel V, Matta MK, Stone MB, Zhu H, Davis MC, Strauss DG, Rouse R. Effects of sedative psychotropic drugs combined with oxycodone on respiratory depression in the rat. Clin Transl Sci. 2021 Nov;14(6):2208-2219. doi: 10.1111/cts.13080
2Xu L, Chockalingam A, Stewart S, Shea K, Matta MK, Narayanasamy S, Pilli NR, Volpe DA, Weaver J, Zhu H, Davis MC, Rouse R. Developing an animal model to detect drug-drug interactions impacting drug-induced respiratory depression. Toxicol Rep. 2020 Jan 25;7:188-197. doi: 10.1016/j.toxrep.2020.01.008
3Florian J, van der Schrier R, Gershuny V, Davis MC, Wang C, Han X, Burkhart K, Prentice K, Shah A, Racz R, Patel V, Matta M, Ismaiel OA, Weaver J, Boughner R, Ford K, Rouse R, Stone M, Sanabria C, Dahan A, Strauss DG. Effect of Paroxetine or Quetiapine Combined With Oxycodone vs Oxycodone Alone on Ventilation During Hypercapnia: A Randomized Clinical Trial. JAMA. 2022 Oct 11;328(14):1405-1414. doi: 10.1001/jama.2022.17735. PMID: 36219407; PMCID: PMC9554704.