Integration of Biorelevant Pediatric Dissolution Methodology into PBPK Modeling to Predict In Vivo Performance and Bioequivalence of Generic Drugs in Pediatric Populations
A Carbamazepine Case Study
CDER researchers recently conducted a study of the anticonvulsant medication carbamazepine (CBZ), to determine the dissolution of this orally administered generic tablet in the gastrointestinal fluids of pediatric patients using in vitro testing methods. Typically, the use of simulated intestinal fluid dissolving methods to test dissolution in human gastrointestinal systems are conducted with adult patients in mind. In this study, CDER researchers looked for potential differences in dissolution profiles based on gastrointestinal (GI) fluid volume, composition, and bile salt concentrations in adult and pediatric patients.
Background
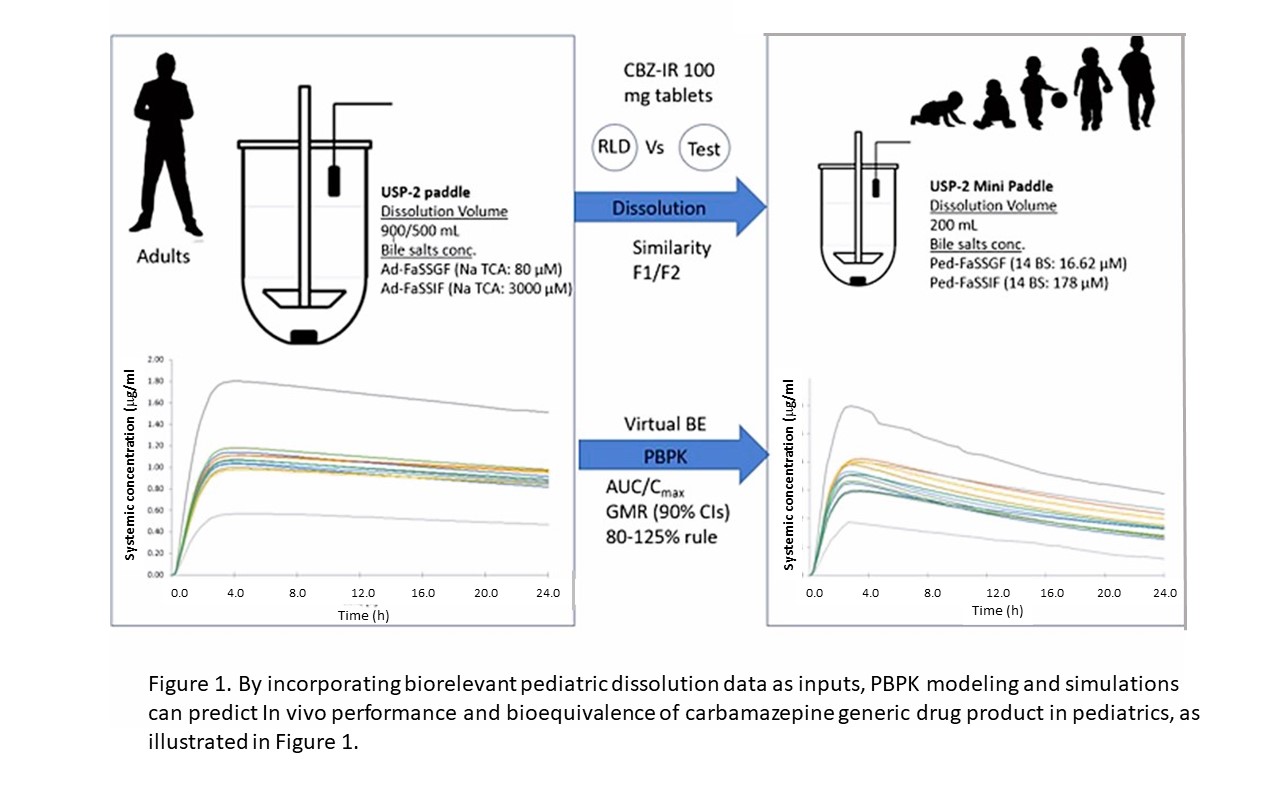
Currently, pediatricians are often faced with the dilemma of whether to prescribe a drug for pediatric patients with only limited evidence-based data of its effect in children and the proper dose. But obtaining this evidence may be challenging. A new drug product developed for children may need separate studies to support its approved use in specific age groups (e.g., neonates, infants, and adolescents) for a variety of reasons, including the determination of the proper dosing to achieve the intended effect in these populations. For generic drugs, generally, a bioequivalence (BE) assessment in adults between generic and reference listed drug product can be used to support a BE assessment in pediatric patients. If the drug product is predominantly intended for use in pediatric patients younger than 6 years of age, a justification is needed that the BE study results obtained from adult subjects are relevant to the pediatric population. Physiologically based pharmacokinetic (PBPK) models can provide a quantitative method for predicting the absorption, distribution, metabolism, and excretion of pharmaceuticals, as well as the drug exposure in adults. When their parameters are updated with information obtained in children, the PBPK predictions can be extrapolated to pediatric populations (1). By incorporating biorelevant pediatric dissolution data as inputs, PBPK modeling and simulations can predict in vivo performance and bioequivalence of carbamazepine generic drug product in pediatrics (Figure 1).
Figure 1. Schematic figure for the development of in vitro dissolution and PBPK modeling to predict in vivo performance and bioequivalence of carbamazepine (CBZ) generic drug product in pediatrics.
The dissolution of tablets in various media is commonly used to help predict the rate of dissolution of orally administered drug products in patients. CDER researchers investigated the impact of GI fluid volume and BS concentrations on the dissolution of CBZ immediate release (IR) 100 mg tablets. CBZ was selected as a model drug in this research because: 1) CBZ is a biopharmaceutics classification system (BCS) class II (poorly soluble; highly permeable) compound where dissolution is a rate limiting factor for its absorption, 2) previous research also found that additional consideration is needed for BCS class II drugs when assessing bioequivalence (BE) in pediatrics (3, 17) moreover, CBZ is a narrow therapeutic index (NTI) drug and caution is required when the dose is bridged from adults to children.
The generated dissolution data were incorporated into a PBPK model to predict the pharmacokinetics (PK) of CBZ in pediatric and adult populations and compared the modeling data to PK data gathered in clinical studies. Dissolution media can be designed to mimic the composition of GI fluids. To mimic the human body’s GI fluids and the dissolving of pills, a fasted state simulated gastric fluid (FaSSGF), or fasted state simulated intestinal fluid (FaSSIF), which are both derived from the characterization and composition of healthy adult GI fluid and secretion media (2,3), were used as dissolution media.
BS concentration in pediatric GI fluids, based on literature reports (4, 5), were found lower in neonates and infants in comparison to adults. Due to the lack of enough accurate data on pediatric BS concentrations, simulated pediatric media with BS concentrations corresponding to 50 and 150% of adult content have been proposed as reasonable for use in exploratory studies (4).
Generally, the recommended dissolution volume has been 500 mL, which most closely resembles the GI volume in adults (6). The volume of fluids in pediatric populations in two recent studies showed fasted gastric volume to be a maximum of 8 mL in a population of children ranging from 0 to 16 years (7), and another study suggested a median volume of 5.0 mL in infants and 26.6 mL in adolescents (6). Corresponding fasted state intestinal fluids have also been reported to reach a maximum of 51 mL in a population of children ranging from 0 to 16 years of age (7) or have a median volume of 23.9 mL in infants and 62.9 mL in adolescents (8). When choosing the dissolution volume to use, an important consideration was the fluid volume administered with a tablet, which is typically 240 mL in adults and usually 120 mL in children (9, 10). In the CDER study, dissolution data generated 500 to 50 mL adult FaSSGF and FaSSIF media as well as 200 mL pediatric FaSSGF and FaSSIF when bile salt media were incorporated into the PBPK model.
Results Discussion
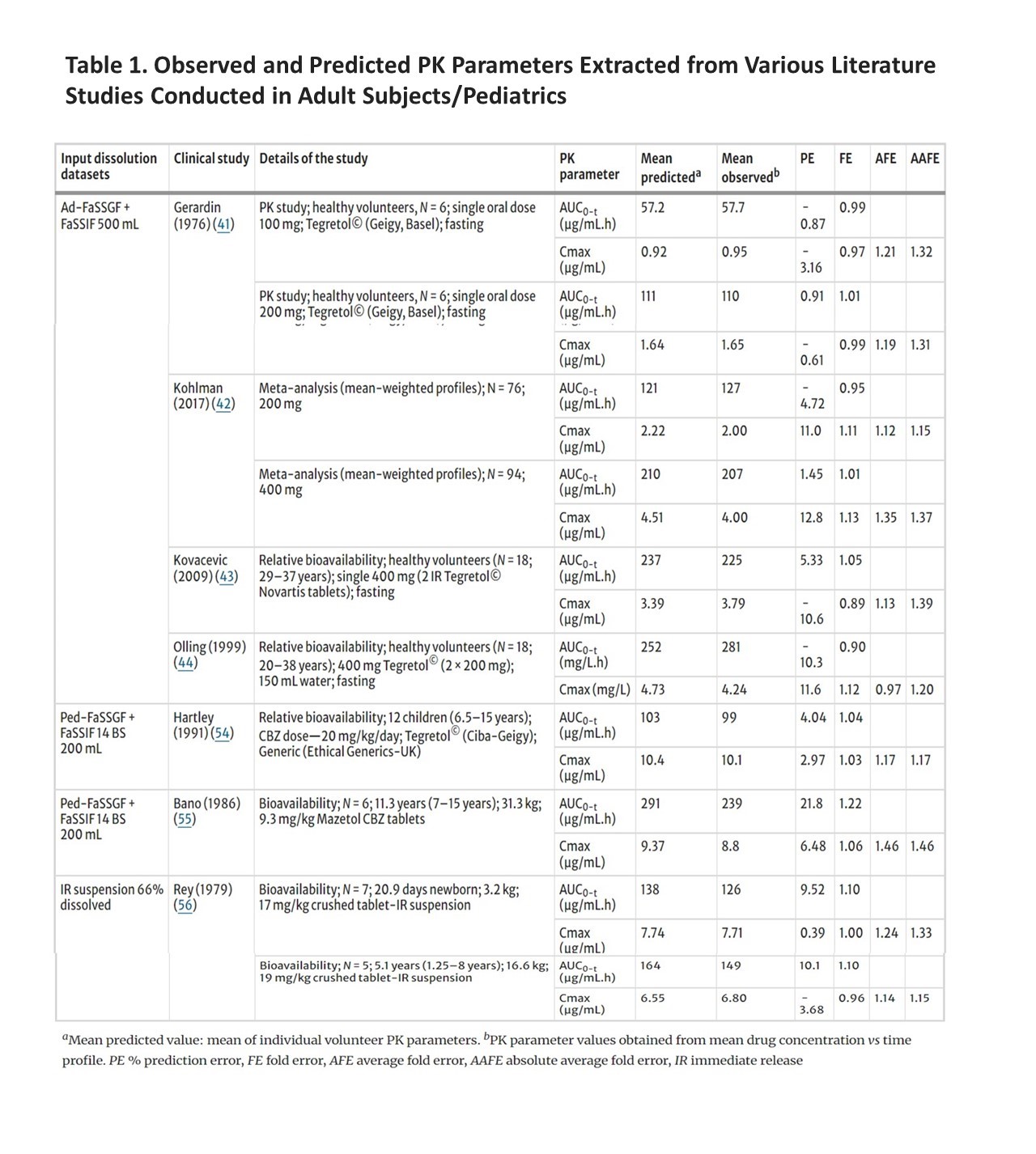
The first objective of this research was to explore the dissolution of CBZ (100 mg)IRtablets by comparing the dissolution in the industry recommended compendial method with the biorelevant dissolving methods. The results suggested complete dissolution of 100 mg CBZ in 1% sodium lauryl sulfate ) at pH 1.2 and 6.5 which agreed with a previous study (11). Comparison of three pediatric media with different composition of bile salts showed similar dissolution profiles of CBZ IR tablets. When these dissolution profiles were incorporated into a validated pediatric PBPK model of CBZ, the model predictions of plasma drug exposure were in close agreement with the clinical data.
In addition, as reported previously, decreasing the dissolution volume (from 900 to 50 mL) to mimic variations in GI fluid volume of pediatric patients aged 0–16 years of age (12) resulted in a decrease in the extent of CBZ dissolution from 91 to 5% in FaSSGF and 94 to 8% in FaSSIF media. This study also showed that the CBZ dissolution was sensitive to changes in volume, a phenomenon that is important to consider when extrapolating from adult to pediatric populations. The validated pediatric PBPK model showed that the dissolution data from 200 mL pediatric FaSSGF and FaSSIF media with 14 bile salts was closest to the pediatric clinical data (13) although a slightly lower volume may have provided a more accurate prediction.
Caution is required when the dose is correlated from adults to children, especially for narrow therapeutic index drugs such as CBZ. Due to the poor solubility of CBZ and low volume of GI fluids in children, there is a risk of precipitation of CBZ along with the excipients leading to sub-therapeutic levels for CBZ. Poorly soluble outcomes were also found in previously published works in which it was observed that CBZ precipitated due to formation of dihydrate crystals with a needle-shaped morphology (12, 14). The researchers prepared the pediatric dissolution media, Ped-FaSSGF and Ped-FaSSIF “fluids” by characterizing the gastric and intestinal fluids from previous research work (12). There are recent reviews (15, 16) providing the details to pediatric GI physiology that could be used to improvise the composition and physicochemical characteristics of pediatric medias, but larger datasets would be needed to further characterize the healthy pediatric GI fluid.
Further, by using different dissolution profiles as model inputs, PBPK modeling demonstrated that the most predictive dissolution volume and media composition to forecast the PK was 500 mL of adult dissolution media, Ad-FaSSGF/Ad-FaSSIF and 200 mL pediatric dissolution media, Ped-FaSSGF/FaSSIF (Table 1). The work presented in this study was limited to a single model drug, CBZ. Additional studies on an expanded set of drug products indicated for use in pediatric patients are needed to better understand and verify biorelevant dissolution data to be used to predict the in vivo performance in pediatric populations.
Table 1. Observed and Predicted PK Parameters Extracted from Various Literature Studies Conducted in Adult Subjects/Pediatrics and Calculated PE, FE, AFE, and AAFE Values
Conclusion
CDER researchers observed that biorelevant GI volumes and fluid composition in dissolution testing provided superior predictions of PK profiles of the dissolving of CBZ in pediatric populations compared to the use of adult biorelevant conditions. The most predictive data for integration into the pediatric PBPK model were those generated using 200 mL pediatric dissolution media (Ped-FaSSGF and Ped FaSSIF), irrespective of bile salt concentration or components used to represent the bile salts. The dissolution profiles in the pediatric dissolution media were close to that of adult FaSSGF and adult FaSSIF 200 mL dissolution levels, and the use of the existing Ad-FaSSGF and Ad-FaSSIF dissolution media may be preferred (at lower volumes) as these are recognized across scientific and regulatory communities and are also readily available. The use of lower dissolution volumes must be considered in the testing and risk assessment of pediatric medicines. However, the work needs to be extended to incorporate additional case studies to determine the most suitable dissolution conditions for a wider range of drug products.
This study demonstrated that the integration of biorelevant dissolution data can predict the PK profile of a poorly soluble drug in both populations. That said, additional studies on an expanded set of drug products indicated for use in pediatric patients are needed to verify biorelevant dissolution data to predict the in vivo performance in pediatrics.
How this research improves generic drug development and supports patient access to needed drugs
Researchers at CDER and the University of Birmingham investigated potential differences in the dissolution profiles of generic tablets of the anticonvulsant carbamazepine based on gastrointestinal fluid volume, composition, and bile salt concentrations in adult and pediatric patients. They demonstrated that integration of the biorelevant dissolution data they obtained in vitro into a physiologically based pharmacokinetic model originally developed in adults could predict the PK profile of carbamazepine, which is a poorly soluble drug in children. This work underscores the potential of such model-informed approaches for assessing relative bioavailability and bioequivalence of generic drugs for pediatric populations in support of pediatric drug development.
(This Impact Story is based on the article by Gopal Pawar, Fang Wu, Liang Zhao, Lanyan Fang, Gilbert J Burckart, Kairui Feng, Youssef M Mousa, Abdullah Al Shoyaib, Marie-Christine Jones, and Hannah K Batchelor, “Integration of Biorelevant Pediatric Dissolution Methodology into PBPK Modeling to Predict In Vivo Performance and Bioequivalence of Generic Drugs in Pediatric Populations: A Carbamazepine Case Study.” AAPS J. 2023 Jun 29;25(4):67.)
References
- Maharaj AR, Edginton AN. Physiologically based pharmacokinetic modeling and simulation in pediatric drug development. CPT Pharmacometrics Syst Pharmacol. 2014;3(11):e150. doi: 10.1038/psp.2014.45.
- Klein S. The use of biorelevant dissolution media to forecast the in vivo performance of a drug. AAPS J. 2010;12(3):397–406.
- Mann J, Dressman J, Rosenblatt K, Ashworth L, Muenster U, Frank K, et al. Validation of dissolution testing with biorelevant media: an OrBiTo study. Mol Pharm. 2017;14(12):4192–201.
- Maharaj AR, Edginton AN, Fotaki N. Assessment of age-related changes in pediatric gastrointestinal solubility. Pharm Res. 2016;33(1):52–71.
- Van der Vossen AC, Hanff LM, Vulto AG, Fotaki N. Potential prediction of formulation performance in paediatric patients using biopharmaceutical tools and simulation of clinically relevant administration scenarios of nifedipine and lorazepam. Br J Clin Pharmacol. 2019;85(8):1728–39.
- Friedel HD, Brown CK, Barker AR, Buhse LF, Keitel S, Kraemer J, et al. FIP Guidelines for dissolution testing of solid oral products. J Pharm Sci. 2018;107(12):2995–3002.
- Papadatou-Soulou E, Mason J, Parsons C, Oates A, Thyagarajan M, Batchelor HK. Magnetic resonance imaging quantification of gastrointestinal liquid volumes and distribution in the gastrointestinal tract of children. Mol Pharm. 2019;16(9):3896–903.
- Van der Veken M, Aertsen M, Brouwers J, Stillhart C, Parrott N, Augustijns P. Gastrointestinal fluid volumes in pediatrics: a retrospective MRI study. Pharmaceutics. 2022;14(9).
- Best BM, Capparelli EV, Diep H, Rossi SS, Farrell MJ, Williams E, et al. Pharmacokinetics of lopinavir/ritonavir crushed versus whole tablets in children. J Acquir Immune Defic Syndr. 2011;58(4):385–91.
- Larsen RH, Hjalgrim LL, Grell K, Kristensen K, Pedersen LG, Brünner ED, et al. Pharmacokinetics of tablet and liquid formulations of oral 6-mercaptopurine in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2020;86(1):25–32.
- El-Massik MA, Abdallah OY, Galal S, Daabis NA. Towards a universal dissolution medium for carbamazepine. Drug Dev Ind Pharm. 2006;32(7):893–905.
- Pawar G, Papadatou-Soulou E, Mason J, Muhammed R, Watson A, Cotter C, et al. Characterisation of fasted state gastric and intestinal fluids collected from children. Eur J Pharm Biopharm. 2021;158:156–65.
- Hartley R, Aleksandrowicz J, Bowmer CJ, Cawood A, Forsythe WI. Dissolution and relative bioavailability of two carbamazepine preparations for children with epilepsy. J Pharm Pharmacol. 1991;43(2):117–9. https:// doi.org/10.1111/j.2042- 7158. 1991. tb066 44.x.
- Murphy D, Rodríguez-Cintrón F, Langevin B, Kelly RC, Rodríguez-Hornedo N. Solution-mediated phase transformation of anhydrous to dihydrate carbamazepine and the effect of lattice disorder. Int J Pharm. 2002;246(1–2):121–34. https://doi.org/10.1016/s0378-5173(02)00358-7.
- Wollmer E, Ungell AL, Nicolas JM, Klein S. Review of paediatric gastrointestinal physiology relevant to the absorption of orally administered medicines. Adv Drug Deliv Rev. 2022;181:114084. https://doi.org/10.1016/j.addr.2021.114084.
- Guimarães M, Statelova M, Holm R, Reppas C, Symilllides M, Vertzoni M, et al. Biopharmaceutical considerations in pediatrics with a view to the evaluation of orally administered drug products - a PEARRL review. J Pharm Pharmacol. 2019;71(4):603–42.
- Pawar G, Wu F, Zhao L, Fang L, Burckart GJ, Feng K, Mousa YM, Naumann F, Batchelor HK. Development of a Pediatric Relative Bioavailability/Bioequivalence Database and Identification of Putative Risk Factors Associated With Evaluation of Pediatric Oral Products. AAPS J. 2021 Apr 21;23(3):57. doi: 10.1208/s12248-021-00592-y.