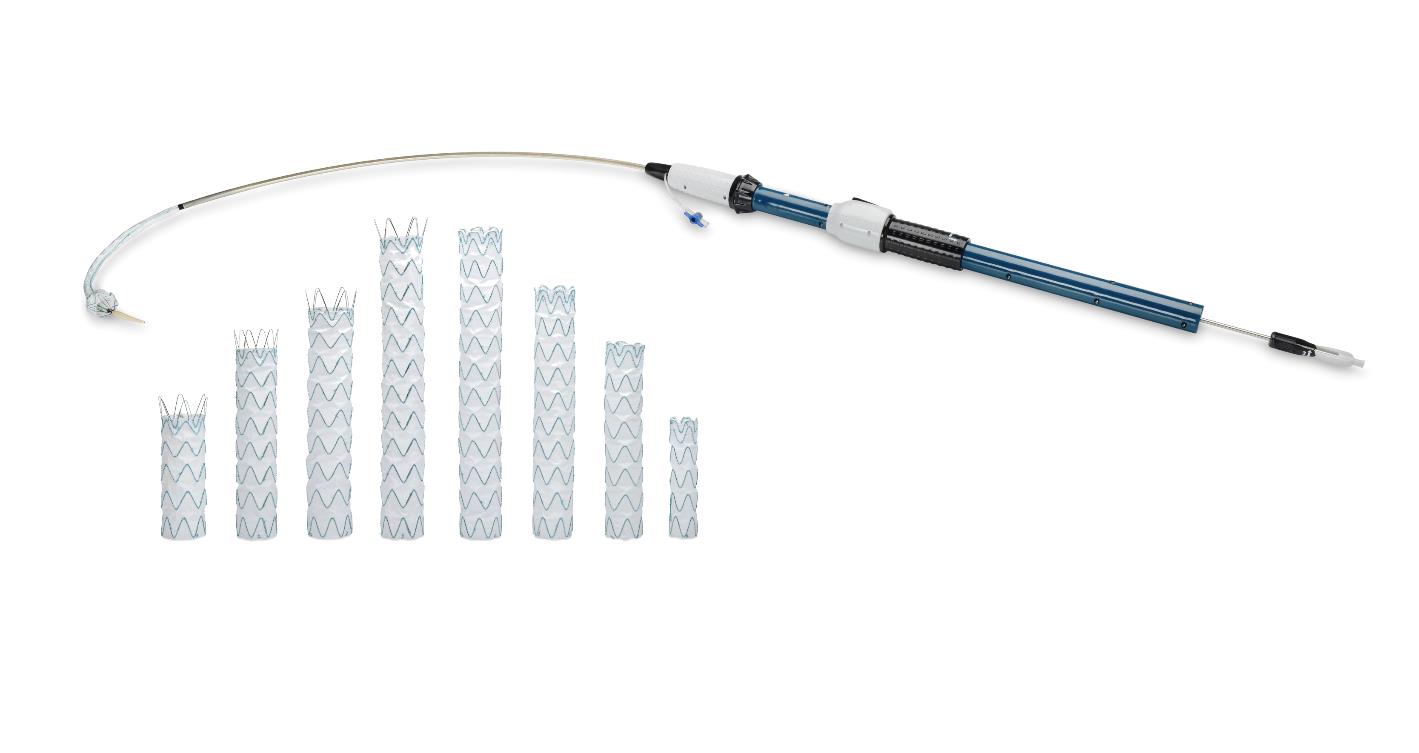
RelayPro Thoracic Stent-Graft System – P200045
This is a brief overview of information related to the FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data and product labeling for more information on this product, its indications for use, and the basis for the FDA’s approval.
Product Name: RelayPro Thoracic Stent-Graft System
PMA Applicant: Bolton Medical, Inc.
Address: 799 International Parkway, Sunrise, FL 33325
Approval Date: August 5, 2021
Approval Letter: Approval Order
What is it?
The RelayPro Thoracic Stent-Graft System is designed to repair a weakened and bulging (aneurysm) section of the largest artery in the body (aorta) in the area behind the heart (descending thoracic aorta). The system consists of a delivery catheter and tube-shaped implants (stent grafts) made of nitinol, polyester, and platinum-iridium. The system has two types of implants, the proximal bare stent, and the non-bare stent (NBS) configurations.
How does it work?
A physician inserts the delivery catheter, carrying the stent graft, into the femoral artery by making a small cut (incision) in the patient’s groin. The delivery catheter is carefully guided to the damaged area behind the heart. Once positioned, the physician removes the delivery catheter, and the stent expands and stays permanently implanted in the artery. This relieves the pressure on the weakened and bulging section of the aorta, which may prevent further aneurysm growth, bursting (rupture) of the aneurysm, and death.
When is it used?
Physicians use the RelayPro Thoracic Stent-Graft System in patients who have aneurysms in the descending thoracic aorta.
What will it accomplish?
The RelayPro Thoracic Stent Graft System is intended to provide a new path for blood flow, which can prevent further aneurysm growth, rupture of the aneurysm, and death.
In a clinical study of 110 patients, 93.6% of patients did not experience major adverse events, such as death, heart attack, respiratory failure, or gut ischemia, up to 30 days after the procedure and 89% were successfully treated without device- or aneurysm-related complications up to one year after the procedure.
When should it not be used?
The RelayPro Thoracic Stent-Graft System should not be used in in patients who have:
- A known allergy or hypersensitivity to devices materials (nitinol, polyester, or platinumiridium).
- A condition that threatens to infect the graft.
Additional information (including warnings, precautions, and adverse events):
- Summary of Safety and Effectiveness Data
- Instructions for Use
- Patient Information Guide
- PMA Database Entry