EPi-Sense® Guided Coagulation System – P200002
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: EPi-Sense® Guided Coagulation System
PMA Applicant: AtriCure, Inc.
Address: 7555 Innovation Way, Mason, OH 45040
Approval Date: April 28, 2021
Approval Letter: Approval Order
What is it?
The EPi-Sense® Guided Coagulation System is a flexible surgical device used to treat long-standing persistent atrial fibrillation, an abnormal heart rhythm (arrythmia) that lasts for more than 12 months. The device is used to destroy a small area of heart tissue on the outside of the heart, called the epicardial surface, that is causing the heart to abnormally beat.
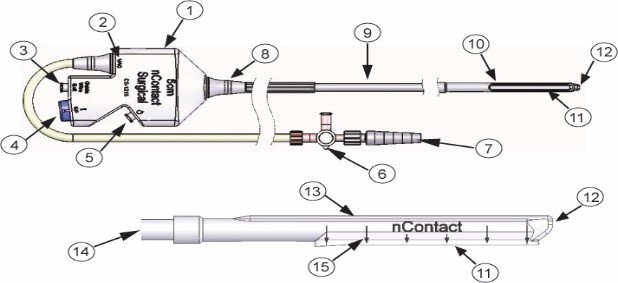
FIG. 1 General Coagulation Device Key Features
(1) Handle; (2) Vacuum Port (3) Guide Wire Exit Port; (4) RF Connection; (5) Perfusion Port; (6) Stopcock; (7) Graduated Fitting to Vacuum Tubing; (8) Strain Relief; (9) Main Body; (10) Distal Shell; (11) Coagulation Electrode and Sensing Electrodes; (12) Guide Tube Opening; (13) Insulative Covering; (14) Vacuum Lumen; (15) Locator Arrows (1cm spacing)
How does it work?
A surgeon makes a surgical cut below the breastbone or in the abdomen and inserts a tube called a cannula to reach the heart. A video camera inside the cannula allows the surgeon to see the heart. The EPi-Sense® Guided Coagulation System is also inserted through the cannula until it reaches the external surface of the left atrium, the heart’s left upper chamber. The device then sends intense pulses of energy (radiofrequency, or RF, energy) to destroy the tissue found on the outside of the heart and that is causing the arrhythmia. The EPi-Sense® Guided Coagulation System is used in combination with another device called an ablation catheter. The catheter is inserted into a blood vessel (vein) in the groin until it reaches the heart. The tip of the catheter delivers heat energy to destroy the areas of heart tissue inside the heart that are causing the abnormal heart rhythm. When the two devices are used together, the procedure is called a “hybrid” procedure because it combines a catheter-based procedure with a surgical procedure. The doctor removes all the devices after treatment.
When is it used?
The EPi-Sense® Guided Coagulation System is combined with an ablation catheter to treat long-standing persistent atrial fibrillation when medications called anti-arrhythmic drugs (AADs) are not working or cannot be tolerated by the patient. The device is only used in patients for whom the benefit of the procedure outweighs its risks, due to the potential for certain complications.
What will it accomplish?
Treatment using the EPi-Sense® Guided Coagulation System in combination with the ablation catheter can prevent episodes of rapid heartbeat or other symptoms caused by the atrial fibrillation.
In a clinical study, doctors treated 38 people with long-standing persistent atrial fibrillation using this device. The heart rhythm was successfully corrected in 25 patients (65.8%) 12 months after the procedure. Three patients (7.9%) experienced complications due to the hybrid procedure that were considered major and may have led to longer hospitalization and/or additional procedures.
Another group of patients were treated with only an ablation catheter. In these patients, heart rhythm was successfully corrected for 37%.
When should it not be used?
The EPi-Sense Guided Coagulation System should not be used in patients who have:
- A clot in the left atrium of the heart (thrombus)
- A systemwide infection
- Active endocarditis, or inflammation of the heart’s inner lining
- Another infection near the site of where surgery will take place
- Barrett’s esophagitis, a condition when the lining of the esophagus is damaged by stomach acid.
Additional information (including warnings, precautions, and adverse events):