Expanded Access (Compassionate Use) Submission Data
CDER, CBER and CDRH Expanded Access INDs and Protocols (2017-2021)
On this page you will find:
- The Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER) expanded access submission receipt reports for INDs and Protocols from 2017-2021
- The Center for Devices and Radiological Health (CDRH) expanded access requests from 2017-2021
The reports are broken down to the following:
FY 2017 – 2021 (most recent 5 years) Graphs of Expanded Access Submissions
To view the graph select the appropriate link below.
For older graphs, see Expanded Access (Compassionate Use) Submission Data Archive: CDER and CBER and Expanded Access (Compassionate Use) Submission Data Archive: CDRH.
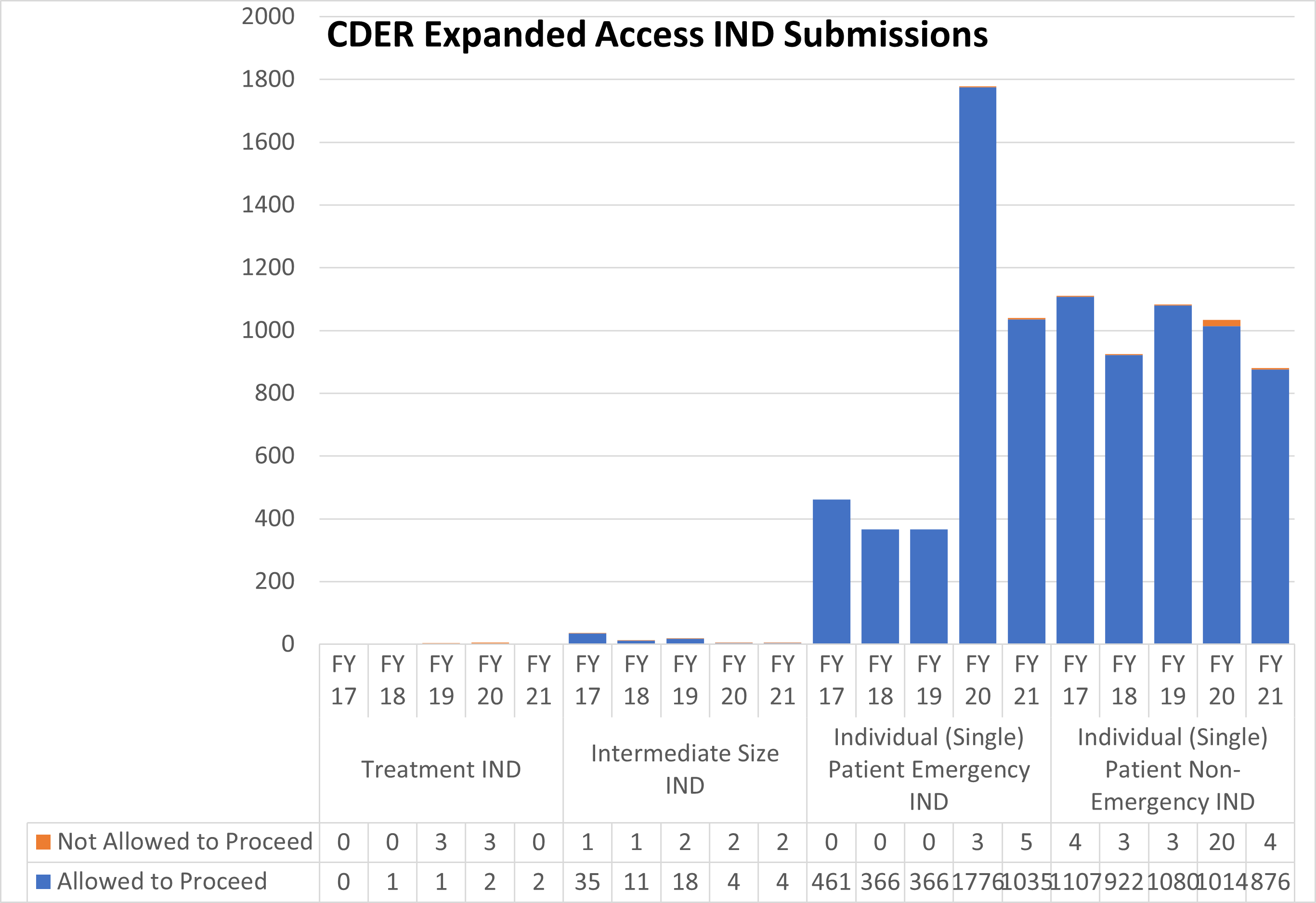
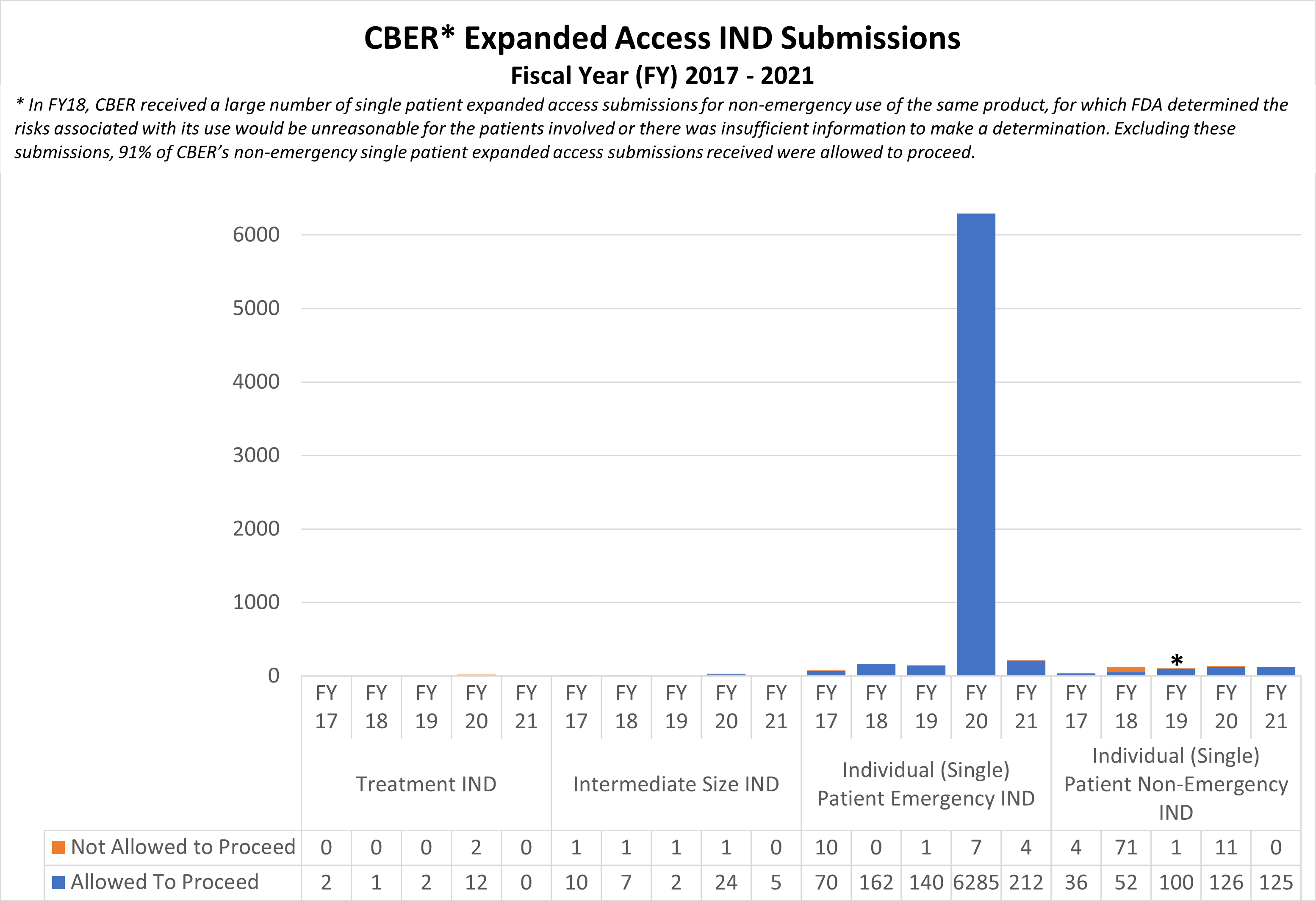
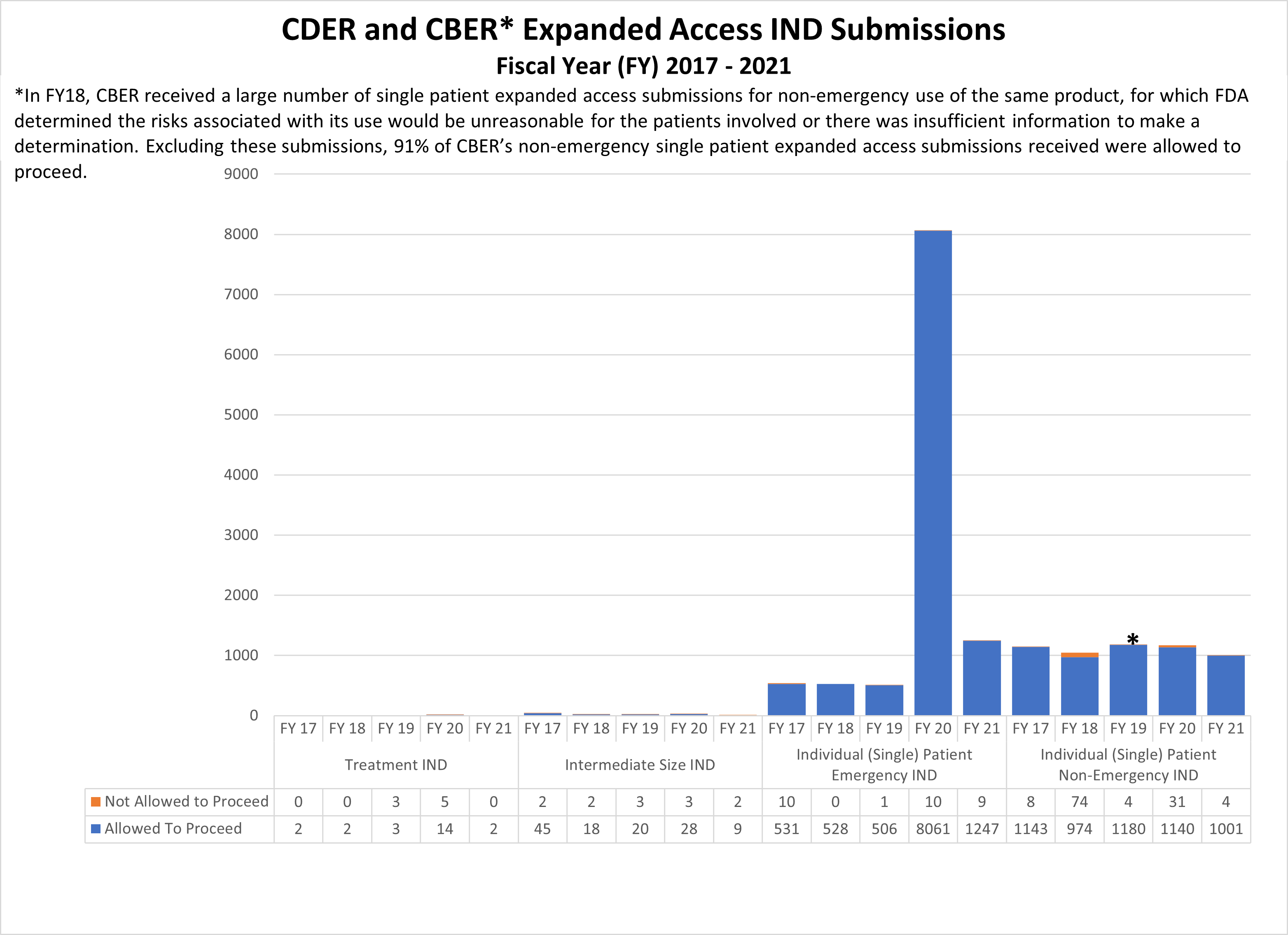
Expanded Access INDs for CDER and CBER (2017-2021)
* In FY18, CBER received a large number of single patient expanded access submissions for non-emergency use of the same product, for which FDA determined the risks associated with its use would be unreasonable for the patients involved or there was insufficient information to make a determination. Excluding these submissions, 91% of CBER’s non-emergency single patient expanded access submissions received were allowed to proceed.
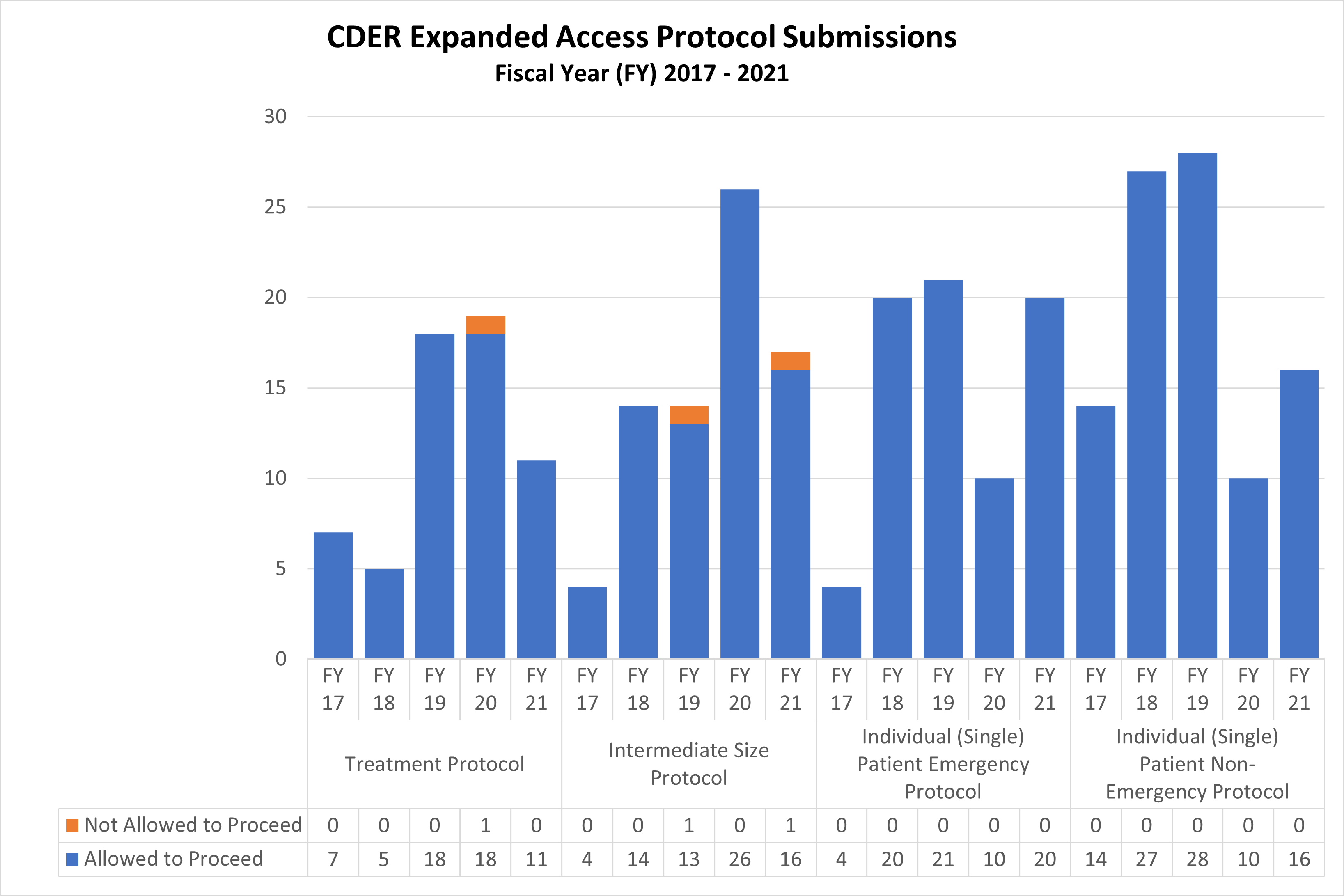
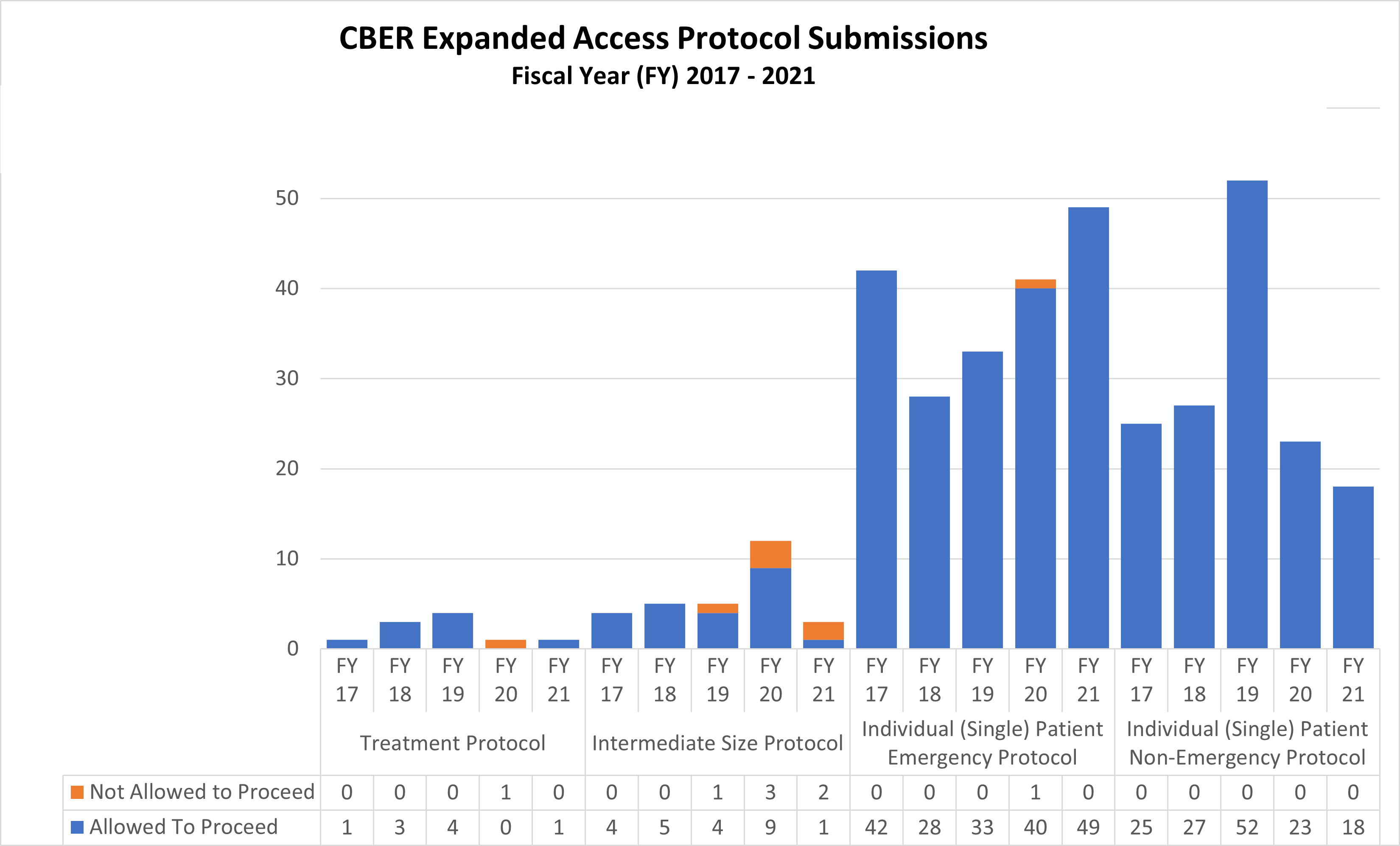
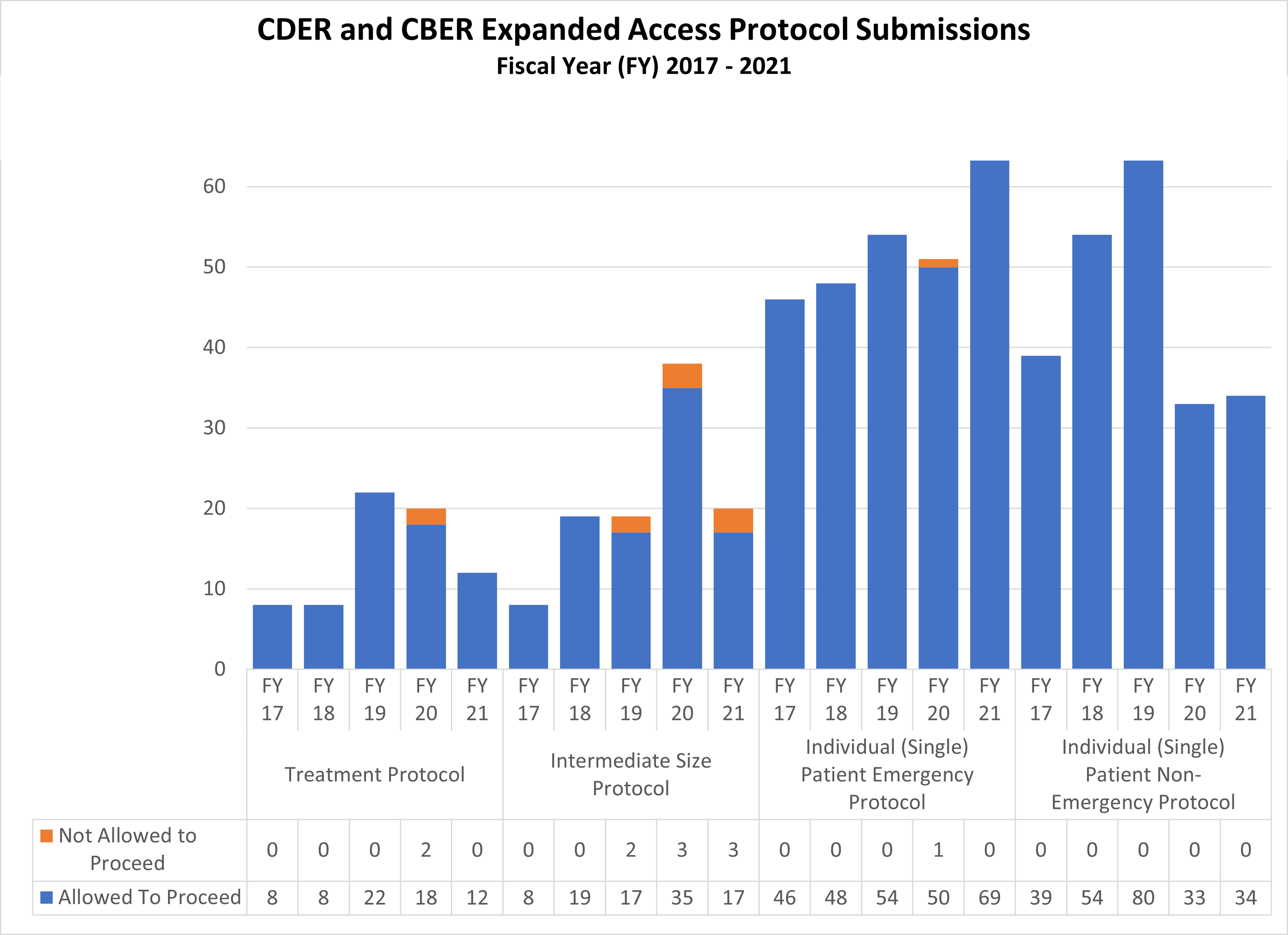
Expanded Access Protocols for CDER and CBER (2017-2021)
FY 2017 – 2021 Graphs of Expanded Access Submissions
CDER Expanded Access INDs and Protocol (2017-2021)
CBER Expanded Access INDs and Protocols (2017-2021)
Combined CDER and CBER Expanded Access Submissions and Protocols (2017-2021)
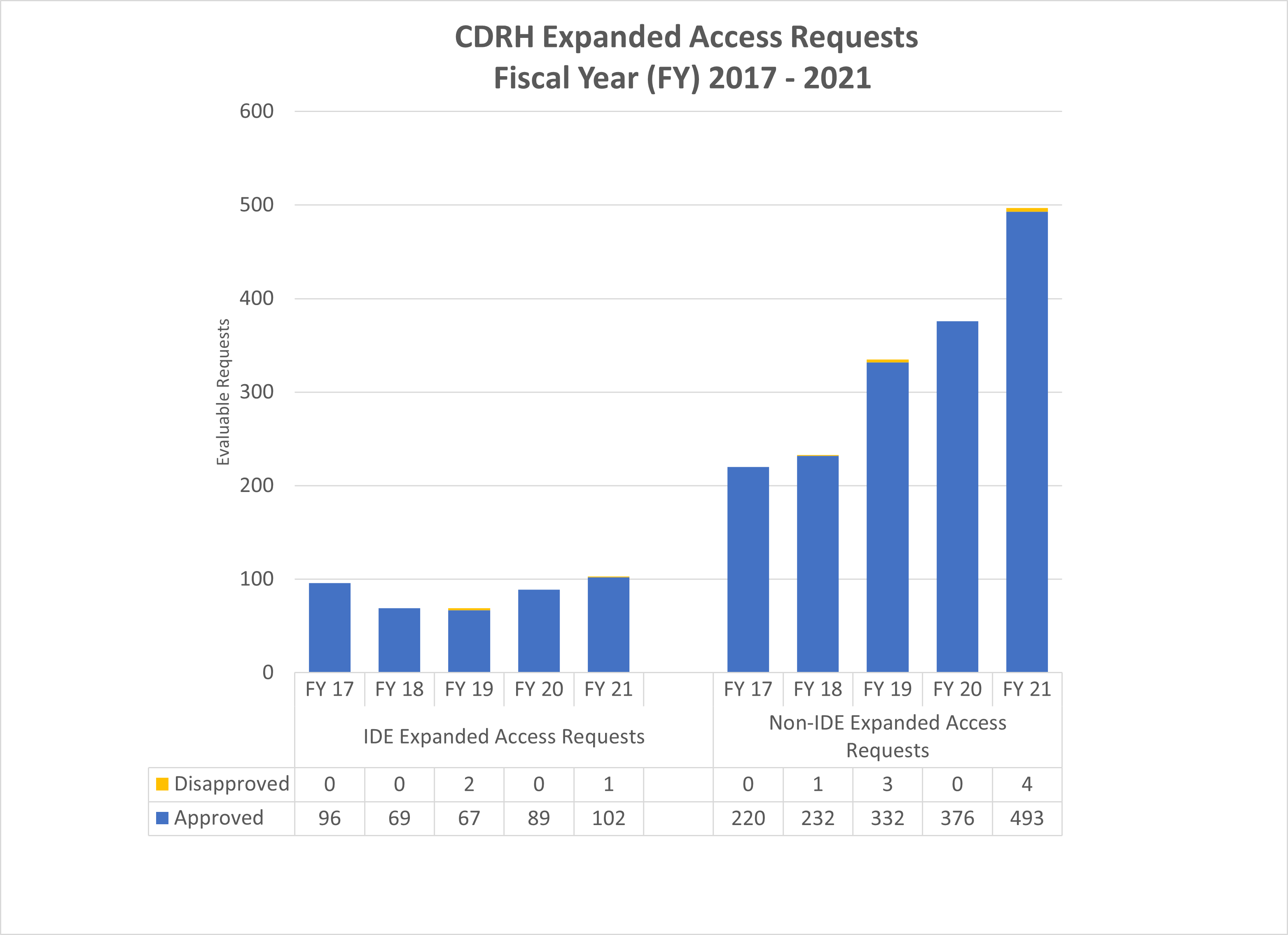
CDRH Expanded Access Requests (2017-2021)
FY 2017-2021 Graph of CDRH Expanded Access Requests
CDRH IDE Expanded Access Requests (2017-2021)
| Expanded Access Totals | FY 2017 | FY 2018 | FY 2019 | FY 2020 | FY 2021 |
|---|---|---|---|---|---|
| Requested Received | 100 | 79 | 76 | 92 | 106 |
| Evaluable Requests | 96 | 69 | 69 | 89 | 103 |
| Approved | 96 | 69 | 67 | 89 | 102 |
| Percentage Approved | 100.0% | 100.0% | 97.1% | 100% | 99.0% |
CDRH Non-IDE Expanded Access Requests(2017-2021)1
| Expanded Access Totals | FY 2017 | FY 2018 | FY 2019 | FY 2020 | FY 2021 |
|---|---|---|---|---|---|
| Requested Received | 230 | 257 | 352 | 384 | 502 |
| Evaluable Requests2 | 220 | 233 | 335 | 372 | 497 |
| Approved | 220 | 232 | 332 | 372 | 493 |
| Percentage Approved3 | 100.0% | 99.6% | 99.1% | 100% | 99.2% |
1 Expanded Access requests outside of an IDE are limited to single patient compassionate use requests.
2 Evaluable requests are requests in which a substantive review was performed on an original, non-IDE compassionate use request resulting in an approval or disapproval decision.
3 Based on approved requests to total number of evaluable requests.