Expanded Access (Compassionate Use) Submission Data Archive: CDER and CBER
Expanded Access (Compassionate Use) Submission Data Archive: CDRH
CDER and CBER Archived Data (2010-2015)
CBER and CDER Tables
FY 2010 – 2015 Graphs of Expanded Access CDER and CBER Submissions
To view the graph select the appropriate link below.
- CDER Expanded Access IND and Protocol Submissions (2010-2015)
- CBER Expanded Access IND and Protocol Submissions (2010-2015)
- Combined CBER and CDER Expanded Access IND and Protocol Submissions (2010-2015)
CBER and CDER Tables
Expanded Access INDs for CDER and CBER (2010-2015)
|
Expanded Access INDs |
Individual (Single) |
Individual (Single) Patient |
Intermediate |
Treatment |
|||||
|---|---|---|---|---|---|---|---|---|---|
| received | allowed to proceed |
received | allowed to proceed |
received | allowed to proceed |
received | allowed to proceed |
||
|
FY 2015 |
CDER |
747 |
745 |
431 |
428 |
46 |
45 |
0 |
0 |
|
CBER |
32 |
29 |
68 |
66 |
2 |
1 |
2 |
2 |
|
|
FY 2014 |
CDER |
696 |
692 |
1069 |
1066 |
52 |
50 |
0 |
0 |
|
CBER |
22 |
19 |
45 |
44 |
1 |
1 |
1 |
1 |
|
|
FY 2013 |
CDER |
550 |
550 |
315 |
313 |
28 |
27 |
0 |
0 |
|
|
CBER |
37 |
31 |
112 |
112 |
10 |
8 |
0 |
0 |
|
FY 2012 |
CDER |
289 |
287 |
14 |
14 |
0 |
0 |
1 |
1 |
|
|
CBER |
47 |
39 |
36 |
35 |
1 |
1 |
2 |
2 |
|
10/13/2010 to 10/12/2011*** |
CDER |
652 |
652 |
443 |
442 |
0 |
0 |
1 |
1 |
|
|
CBER |
22 |
18 |
24 |
24 |
4 |
4 |
1 |
1 |
|
10/13/2009 to 10/12/2010*** |
CDER |
484 |
484 |
516 |
500 |
2 |
2 |
0 |
0 |
|
|
CBER |
17 |
14 |
24 |
24 |
1 |
1 |
0 |
0 |
*** These reporting periods cover a one-year cohort starting the day the Final Rule for Expanded Access to Investigational Drugs for Treatment Use and Charging for Investigational Drugs went into effect. Starting with Fiscal Year 2012, the reporting period was changed to a fiscal year to match the reporting period for other IND activity reports.
Expanded Access Protocols for CDER and CBER (2010-2015)
|
|
Individual (Single) |
Individual (Single) Patient |
Intermediate |
Treatment |
|||||
|---|---|---|---|---|---|---|---|---|---|
| received | allowed to proceed |
received | allowed to proceed |
received | allowed to proceed |
received | allowed to proceed |
||
|
FY 2015 |
CDER |
14 |
14 |
7 |
7 |
9 |
9 |
8 |
8 |
|
CBER |
48 |
47 |
13 |
13 |
1 |
0 |
2 |
2 |
|
|
FY 2014 |
CDER |
35 |
35 |
9 |
9 |
9 |
9 |
12 |
12 |
|
CBER |
33 |
32 |
22 |
22 |
5 |
5 |
2 |
2 |
|
|
FY 2013 |
CDER |
62 |
62 |
2 |
2 |
8 |
8 |
12 |
12 |
|
|
CBER |
56 |
56 |
14 |
14 |
7 |
5 |
0 |
0 |
|
FY 2012 |
CDER |
121 |
121 |
0 |
0 |
8 |
8 |
10 |
10 |
|
|
CBER |
49 |
47 |
19 |
19 |
3 |
2 |
0 |
0 |
|
10/13/2010 to 10/12/2011*** |
CDER |
89 |
89 |
3 |
3 |
1 |
1 |
11 |
11 |
|
|
CBER |
36 |
36 |
6 |
6 |
4 |
4 |
1 |
1 |
|
10/13/2009 to 10/12/2010*** |
CDER |
16 |
16 |
0 |
0 |
5 |
5 |
7 |
7 |
|
|
CBER |
31 |
30 |
4 |
4 |
1 |
1 |
0 |
0 |
*** These reporting periods cover a one-year cohort starting the day the Final Rule for Expanded Access to Investigational Drugs for Treatment Use and Charging for Investigational Drugs went into effect. Starting with Fiscal Year 2012, the reporting period was changed to a fiscal year to match the reporting period for other IND activity reports.
FY 2010 – 2015 Graphs of Expanded Access Submissions
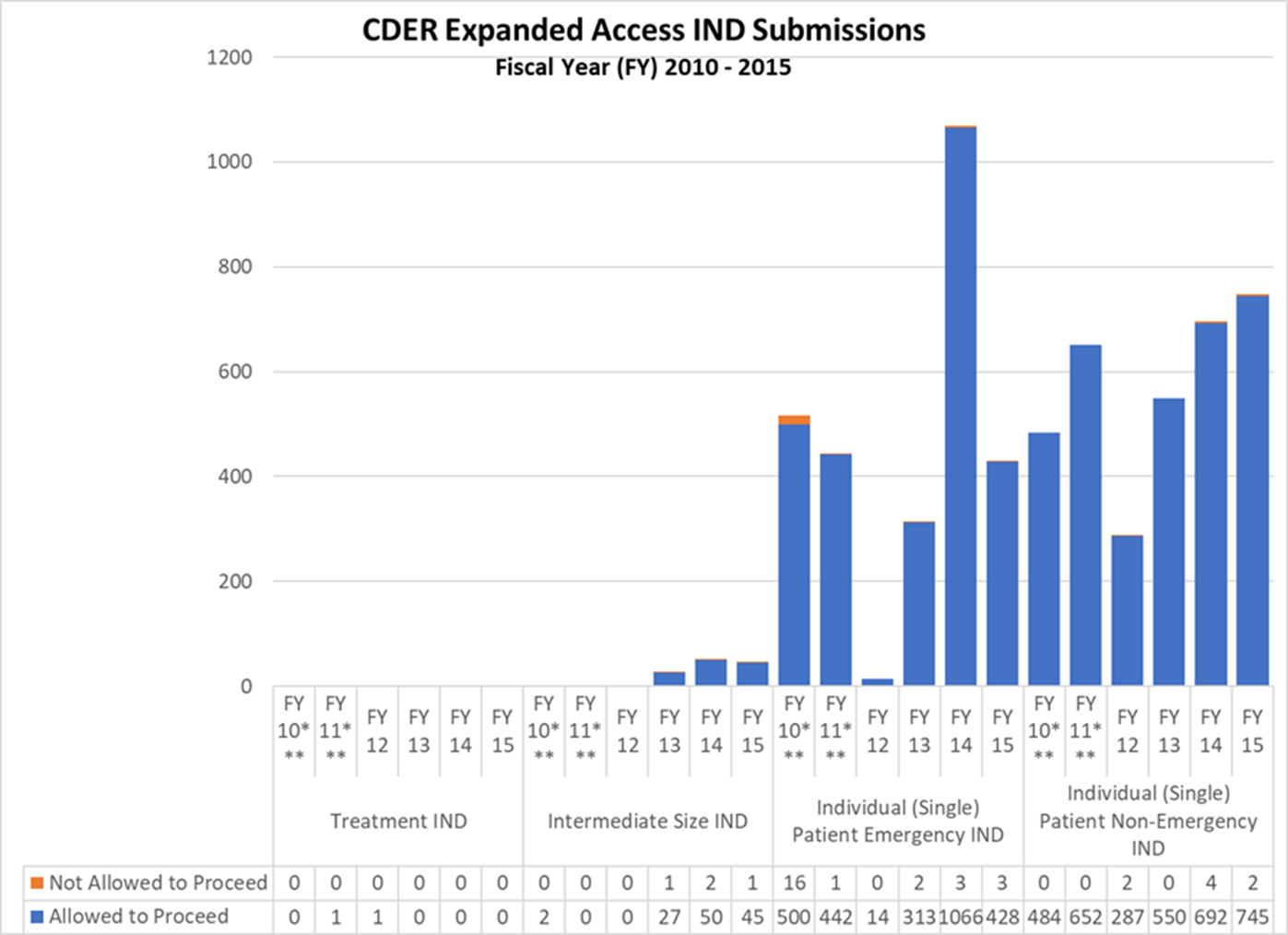
CDER Expanded Access IND and Protocol Submissions (2010-2015)
*** These reporting periods cover a one-year cohort starting the day the Final Rule for Expanded Access to Investigational Drugs for Treatment Use and Charging for Investigational Drugs went into effect. Starting with Fiscal Year 2012, the reporting period was changed to a fiscal year to match the reporting period for other IND activity reports.
CBER Expanded Access IND and Protocol Submissions (2010-2015)
*** These reporting periods cover a one-year cohort starting the day the Final Rule for Expanded Access to Investigational Drugs for Treatment Use and Charging for Investigational Drugs went into effect. Starting with Fiscal Year 2012, the reporting period was changed to a fiscal year to match the reporting period for other IND activity reports.
Combined CBER and CDER Expanded IND and Protocol Submissions (2010-2015)
*** These reporting periods cover a one-year cohort starting the day the Final Rule for Expanded Access to Investigational Drugs for Treatment Use and Charging for Investigational Drugs went into effect. Starting with Fiscal Year 2012, the reporting period was changed to a fiscal year to match the reporting period for other IND activity reports.