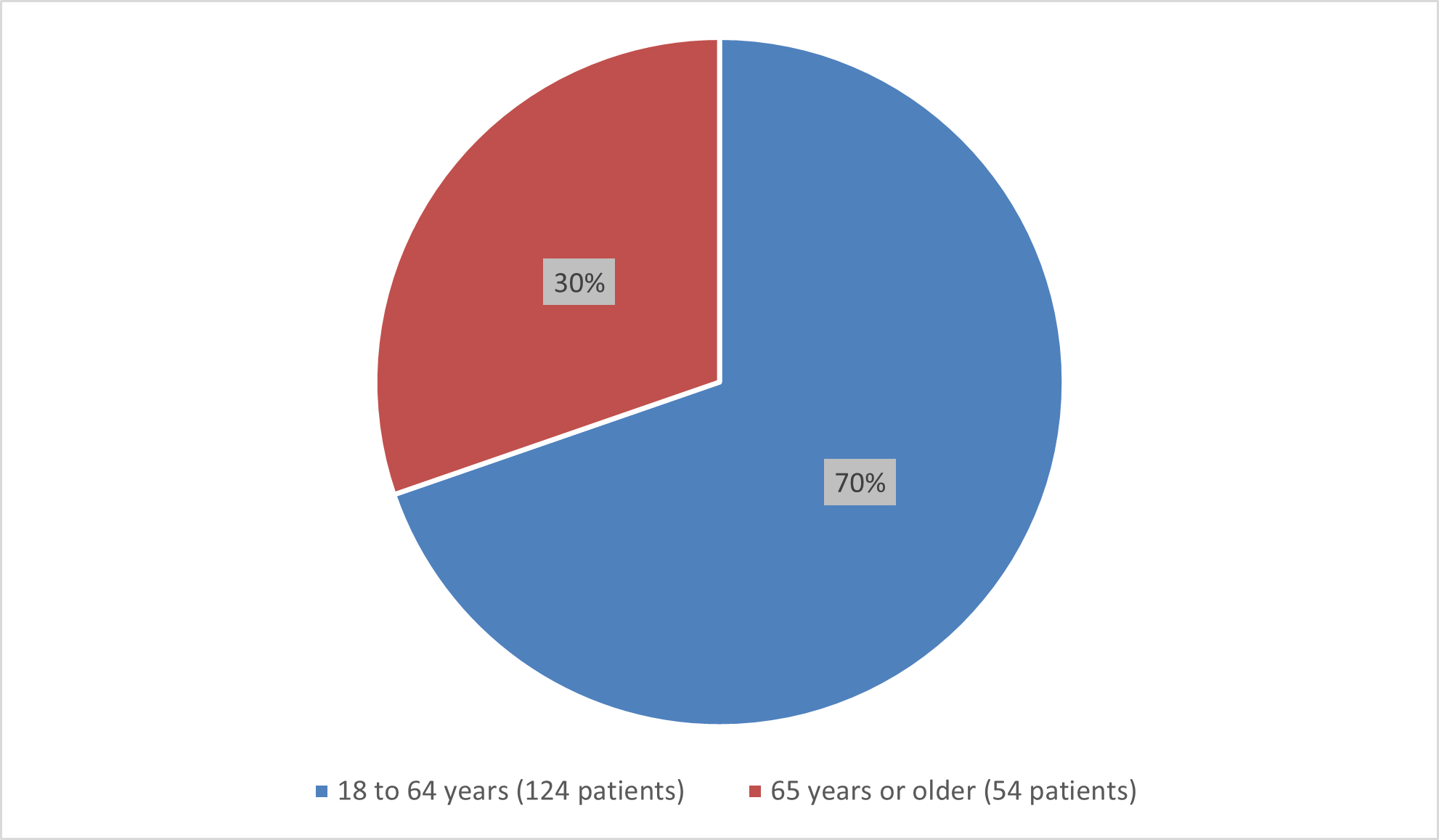Drug Trials Snapshots: BESREMi
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the BESREMi Prescribing Information (USPI for BESREMi®) for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
BESREMi (ropeginterferon alfa-2b-njft) injection
(bez-reh-me)
PharmaEssentia Corporation
Approval date: November 12, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
BESREMi is an interferon alfa-2b drug used to treat adults with polycythemia vera, a blood disease that causes the overproduction of red blood cells. The excess cells thicken the blood, slowing blood flow and increasing the chance of blood clots.
How is this drug used?
How is this drug used? BESREMi is an injection that is given under the skin (subcutaneously) every three weeks. The starting dosage may differ for adults with polycythemia vera who are not on hydroxyurea or transitioning from hydroxyurea, known as the titration phase (refer to the BESREMi Prescribing Information: USPI for BESREMi®).
Who participated in the clinical trials?
The FDA approved BESREMi based on evidence from a clinical trial of 51 patients with polycythemia vera. The trial was conducted at six sites exclusively in Austria. The safety of BESREMi was based primarily on findings from the efficacy study in 51 patients. Two additional studies served as supportive safety data; therefore, the number of patients representing efficacy findings may differ from the number of patients representing safety findings due to different pools of study participants analyzed for efficacy and safety.
Who participated in the trials?
Table 1. Other Demographic Characteristics of the PEGINVERA Study All-treated Population
| Demographic Characteristic | Ropeginterferon Alfa-2b N=51 |
|---|---|
| Disease history | |
| Known disease | 43 (84.3%) |
| Newly diagnosed | 8 (15.7%) |
| Duration of PV (months) from diagnosis at screening, mean [SD] | 51.21 [57.12] |
| JAK 2 allelic burden at baseline | |
| Count subjects with data | 51 |
| Mean [SD] | 44.35 [28.67] |
| Median | 42.0 |
| Range | 0-99 |
| Normal spleen size at baseline, n (%) | N=50 |
| No | 16 (31.4%) |
| Yes | 30 (60.0%) |
| Missing | 4 (8%) |
| Spleen size at baseline, males (≤13 cm), n (%) | N=31 |
| Normal | 13 (41.9%) |
| Enlarged | 16 (51.6%) |
| Missing | 2 (6.5%) |
| Spleen size at baseline, females (≤12 cm), n (%) | N=20 |
| Normal | 6 (30.0%) |
| Enlarged | 12 (60.0%) |
| Missing | 2 (10.0%) |
| Treatment with hydroxyurea at baseline | N=51 |
| No | 34 (66.7%) |
| Yes | 17 (33.3%) |
| Number of phlebotomies in last 3 months prior to screening (mean ± SD) | 1.5±1.9 |
| Number of phlebotomies from screening to baseline, n (%) | |
| 0 | 27 (52.9%) |
| 1 | 14 (27.5%) |
| 2 | 5 (9.8%) |
| 3 | 4 (7.8%) |
| 6 | 1 (2.0%) |
| Hematocrit at baseline (%) | 45.1±4.00 |
| Platelets at baseline (109/L) | 457.9±186.5 |
| Leukocytes at baseline (109/L) | 11.8±5.2 |
Source: Adapted from FDA review
Abbreviations: PV, polycythemia vera; SD, standard deviation
What are the benefits of this drug?
BESREMi works to normalize blood counts, spleen size, and prevent new blood clots. In the trial, 61% of patients receiving BESREMi achieved this benefit (see the BESREMi Prescribing Information: USPI for BESREMi®).
What are the benefits of this drug (results of trials used to assess efficacy)?
It took at least 14 months to achieve a response of normalizing blood counts, spleen size, and prevention of new blood clots with the use of BESREMi.
Table 2. Duration of Response for CHR
| Parameter | Total N=51 |
|---|---|
| Subjects with response, n (%) | 31 (60.8%) |
| Duration of longest response, months | |
| Events, n (%) | 21 (67.7%) |
| Censored, n (%) | 10 (32.3%) |
| No PD/No death | 8 (25.8%) |
| PD/Death after 2 or more missed assessments | 2 (6.5%) |
| 25th Percentile (95% CI) | 3.70 (2.10, 8.52) |
| Median (95% CI) | 14.26 (5.54, 30.10) |
| 75th Percentile (95% CI) | 43.87 (16.56, NC) |
| Min, max | 61.25 |
Source: Adapted from FDA Review
Abbreviations: CHR, complete hematological response; CI, confidence interval; PD, pharmacodynamics; NC, not calculated
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
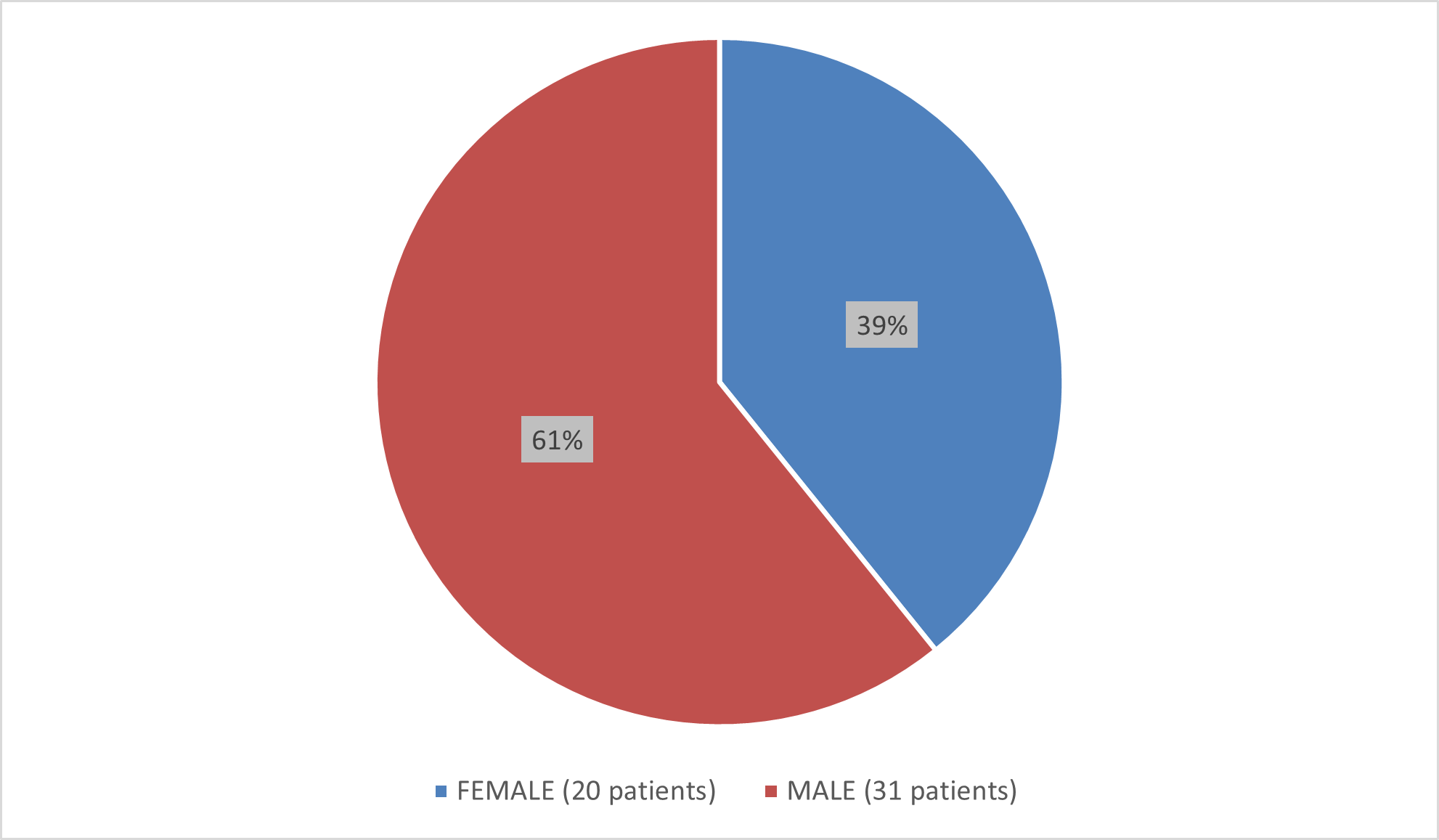
- Sex: BESREMi worked similarly in female and male subjects regarding complete hematological response (CHR). More male subjects were treated and completed the study than female subjects.
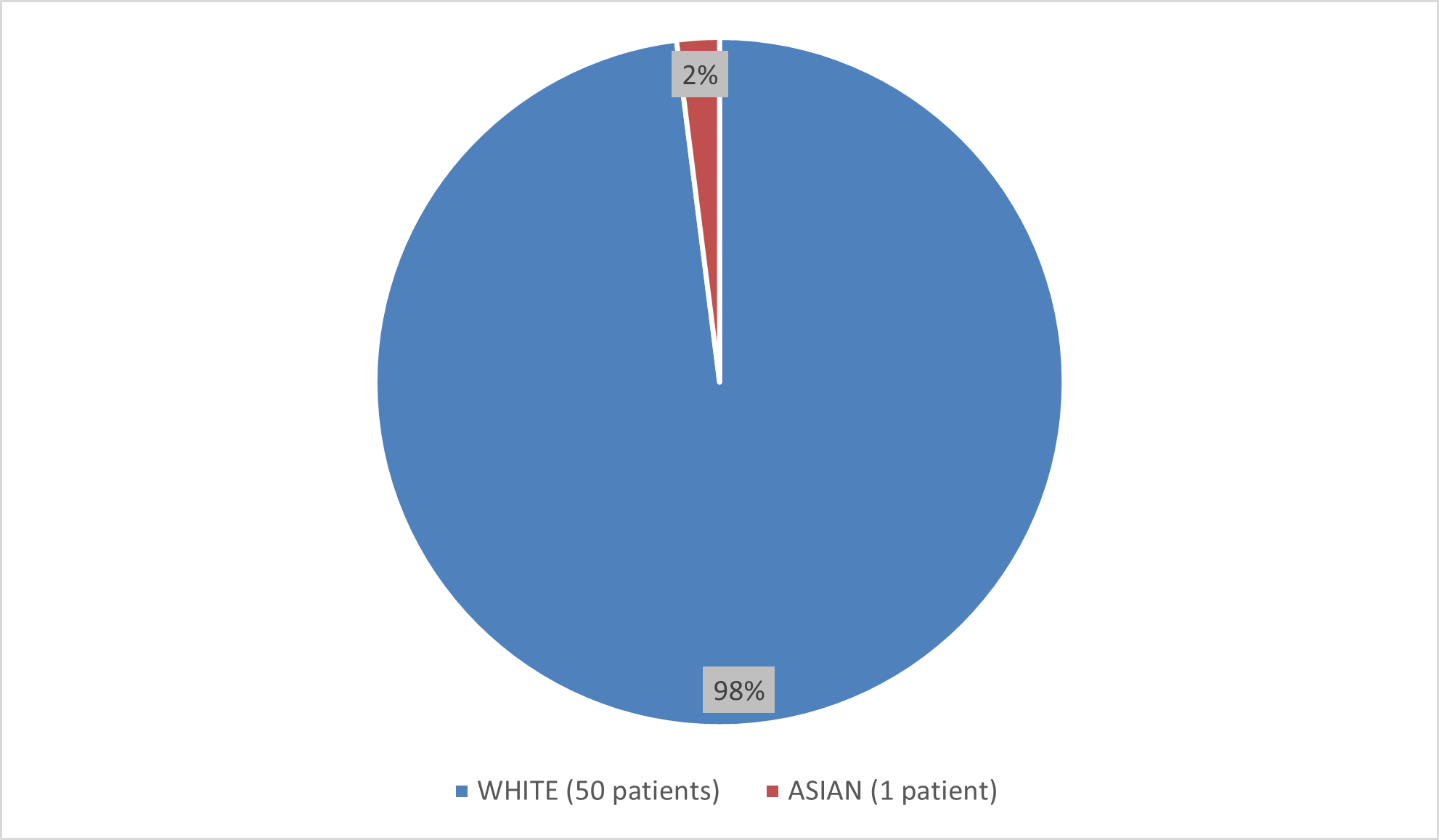
- Race: The number of patients of races other than White was very small; therefore, differences in how BESREMi worked among races could not be determined.
- Age: BESREMi worked similarly in patients below and above 65 years of age regarding CHR.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3. Subgroup Analysis of CHR
| Demographic Characteristics | n/N | CHR Rate (95%CI) |
|---|---|---|
| Sex | ||
| Female | 11/20 | 55% (32, 77) |
| Male | 20/31 | 65% (45, 81) |
| Age | ||
| 18 to <65 years | 19/34 | 56% (38, 73) |
| ≥65 years | 12/17 | 71% (44, 90) |
Source: Adapted from FDA Review
Abbreviations: CHR, complete hematological response; CI, confidence interval
What are the possible side effects?
BESREMi can cause serious side effects including urinary tract infection, transient ischemic attack, and depression. BESREMi may cause harm to unborn babies. The most common side effects of BESREMi are liver enzyme elevation, leukopenia, thrombocytopenia, arthralgia, fatigue, myalgia, and influenza-like illness. Monitor complete blood count more frequently if clinically indicated. Phlebotomy as rescue treatment to normalize blood hyperviscosity may be necessary during the titration phase.
Warnings and Precautions listed below have occurred in patients receiving products similar to BESREMi, some of which were identified during the use of BESREMi. They include depression and suicide, endocrine toxicity, cardiovascular toxicity, decreased peripheral blood counts, pancreatitis, ophthalmologic toxicity, hyperlipidemia, liver and renal toxicities (USPI for BESREMi®).
What are the possible side effects (results of trials used to assess safety)?
Table 4. Adverse Reactions in >10% of Subjects With Polycythemia Vera in the PEGINVERA Study Over 7.5 Years
| Adverse Reactions* | BESREMi N=51 % |
|---|---|
| Influenza-like illnessa | 59 |
| Arthralgia | 47 |
| Fatigueb | 47 |
| Pruritis | 45 |
| Nasopharyngitisc | 43 |
| Musculoskeletal paind | 41 |
| Headachee | 39 |
| Diarrhea | 33 |
| Hyperhidrosisf | 29 |
| Nausa | 28 |
| Upper respiratory tract infectiong | 27 |
| Local administration site reactions | 26 |
| Dizziness | 22 |
| Abdominal painh | 20 |
| Depression | 20 |
| Sleep disorderi | 20 |
| Leukopenia | 18 |
| Decreased appetite | 18 |
| Alopecia | 16 |
| Edemaj | 16 |
| Hypertensionk | 16 |
| Muscle spasms | 16 |
| Neutropenia | 16 |
| Rashl | 16 |
| Transaminase elevationsm | 16 |
| Urinary tract infection | 16 |
| Thrombocytopenia | 12 |
| Vertigo | 12 |
Source: Adapted from FDA Review
* Adverse reactions defined as all treatment emergent adverse events.
a Includes pyrexia, chills, and influenza-like illness.
b Includes asthenia, malaise, and fatigue.
c Includes pharyngitis and nasopharyngitis.
d Includes musculoskeletal pain, back pain, pain in extremity, bone pain, flank pain, and spinal pain.
e Includes headache, migraine, and head pain.
f Includes night sweats and hyperhidrosis.
g Includes upper respiratory tract infection, rhinitis, bronchitis, and respiratory tract infection.
h Includes abdominal pain upper, abdominal pain lower, and abdominal pain.
i Includes insomnia, sleep disorder, and abnormal dreams.
j Includes peripheral edema and generalized edema.
k Includes hypertension and hypertensive crisis.
l Includes rash, maculopapular rash, and pruritic rash.
m Includes transaminase increase, hepatic enzyme increase, GGT increase, AST increase, and ALT increase.
Clinically relevant adverse reactions in <10% of patients include: Cardiovascular system – atrial fibrillation.
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase side effect
Were there any differences in side effects among sex, race and age?
- Sex: The rate of side effects from BESREMi was similar in both females and males. The rate of Grade 3 or higher side effects from BESREMi was slightly larger among females.
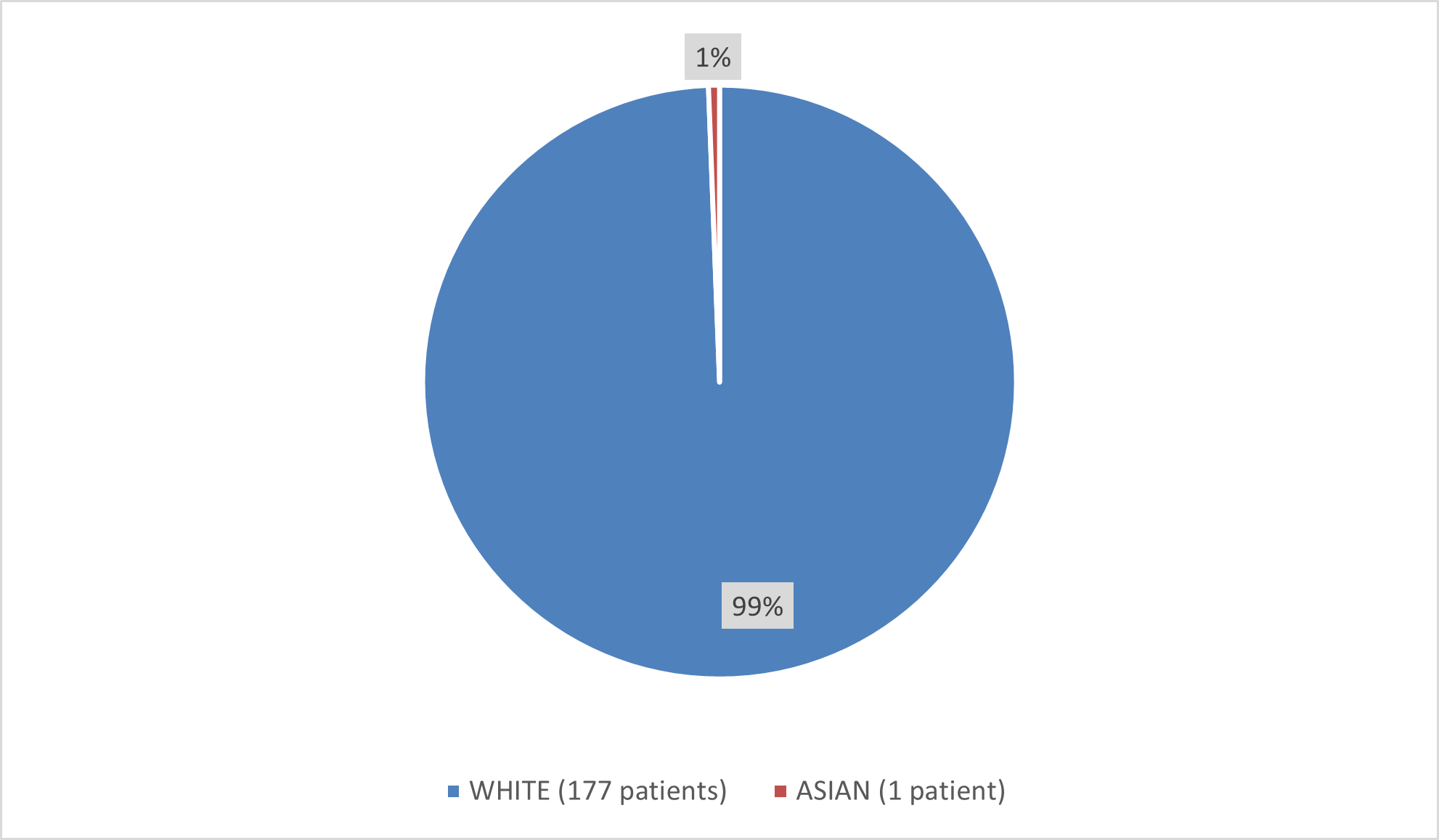
- Race: The number of patients of races other than White was small; therefore, differences among races in the rate of side effects from BESREMi could not be determined
- Age: The rate of side effects was slightly larger in the >=65-year-old age group compared to the 18–64-year-old group. >=65-year-old age group.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 5. Overview of Side Effects by Sex, Race, and Age In Patients With Polycythemia Vera Who Received BESREMi In the PEGINVERA Study
| Demographic Variable | BESREMi N=51 |
|
|---|---|---|
| All Grades n/Ns (%) |
Grades 3 to 5 n/Ns (%) |
|
| Sex | ||
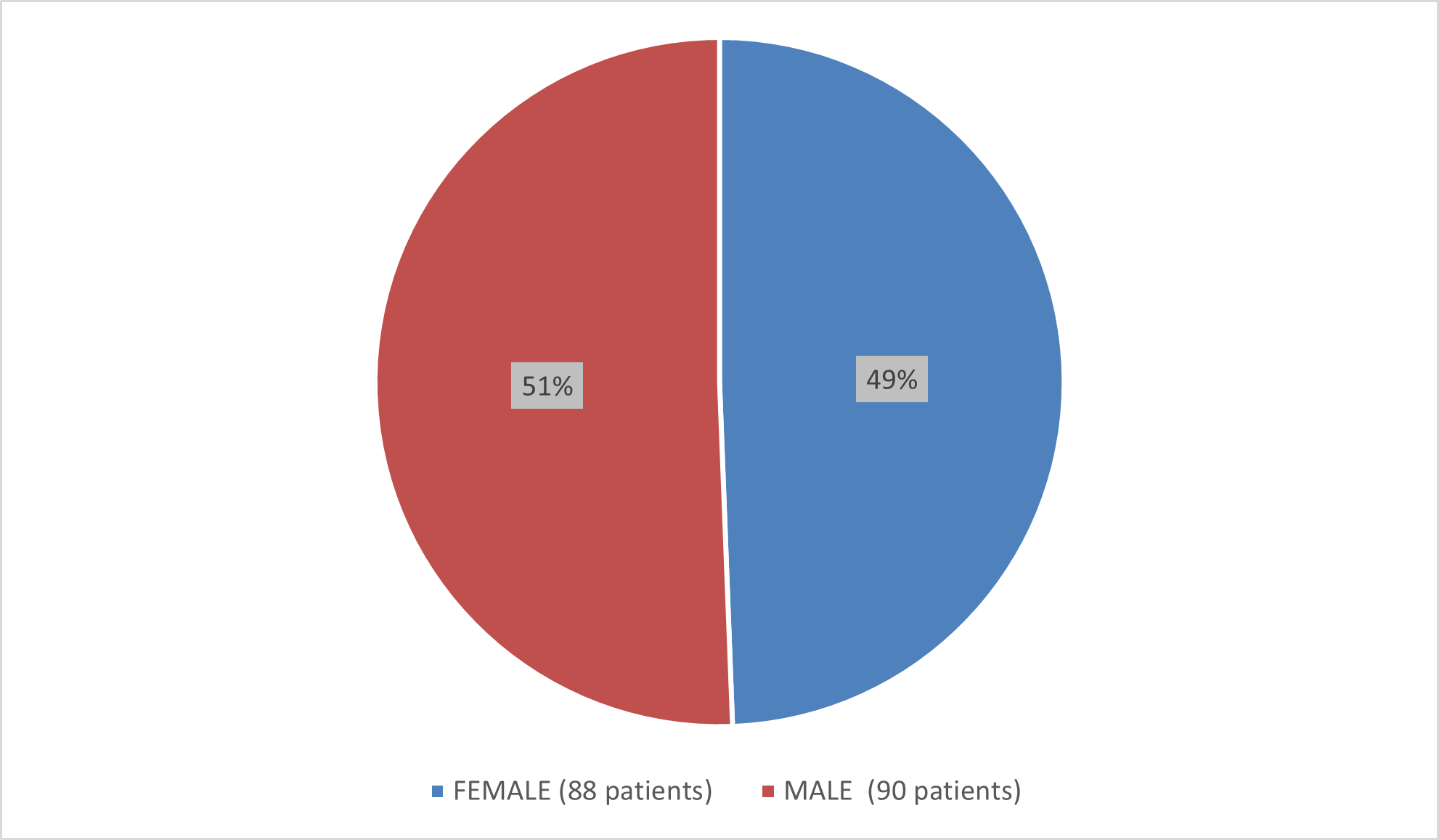
| Female | 20/20 (100) | 12/20 (60.0) |
| Male | 31/31 (100) | 14/31 (45.2) |
| Race | ||
| Asian | 1/1 (100) | 0/1 (0) |
| White | 50/50 (100) | 26/50 (52.0) |
| Age group | ||
| 18 to <65 years | 34/34 (100) | 16/34 (47.1) |
| ≥65 years | 17/17 (100) | 10/17 (58.8) |
Source: FDA reviewer’s analysis
Abbreviation: N, number of patients in the safety population; n, number of patients with given characteristic; Ns, total number of patients in each category
DEMOGRAPHICS SNAPSHOT
Figure 1 summarizes how many male and female patients were enrolled in the clinical trials used to evaluate the efficacy of BESREMi.
Figure 1. Baseline Demographics by Sex (Efficacy Population)
Source: Adapted from FDA Review
Figure 2 summarizes how many patients by sex were in the combined trials used to evaluate the side effects of BESREMi.
Figure 2. Baseline Demographics by Sex (Safety Population)
Source: Adapted from FDA Review
Figure 3 summarizes the percentage of patients by race enrolled in the clinical trial used to evaluate the efficacy of BESREMi.
Figure 3. Baseline Demographics by Race (Efficacy Population)
Source: Adapted from FDA Review
Figure 4 summarizes how many patients by race were in the combined trials used to evaluate the side effects of BESREMi.
Figure 4. Baseline Demographics by Race (Safety Population)
Source: Adapted from FDA Review
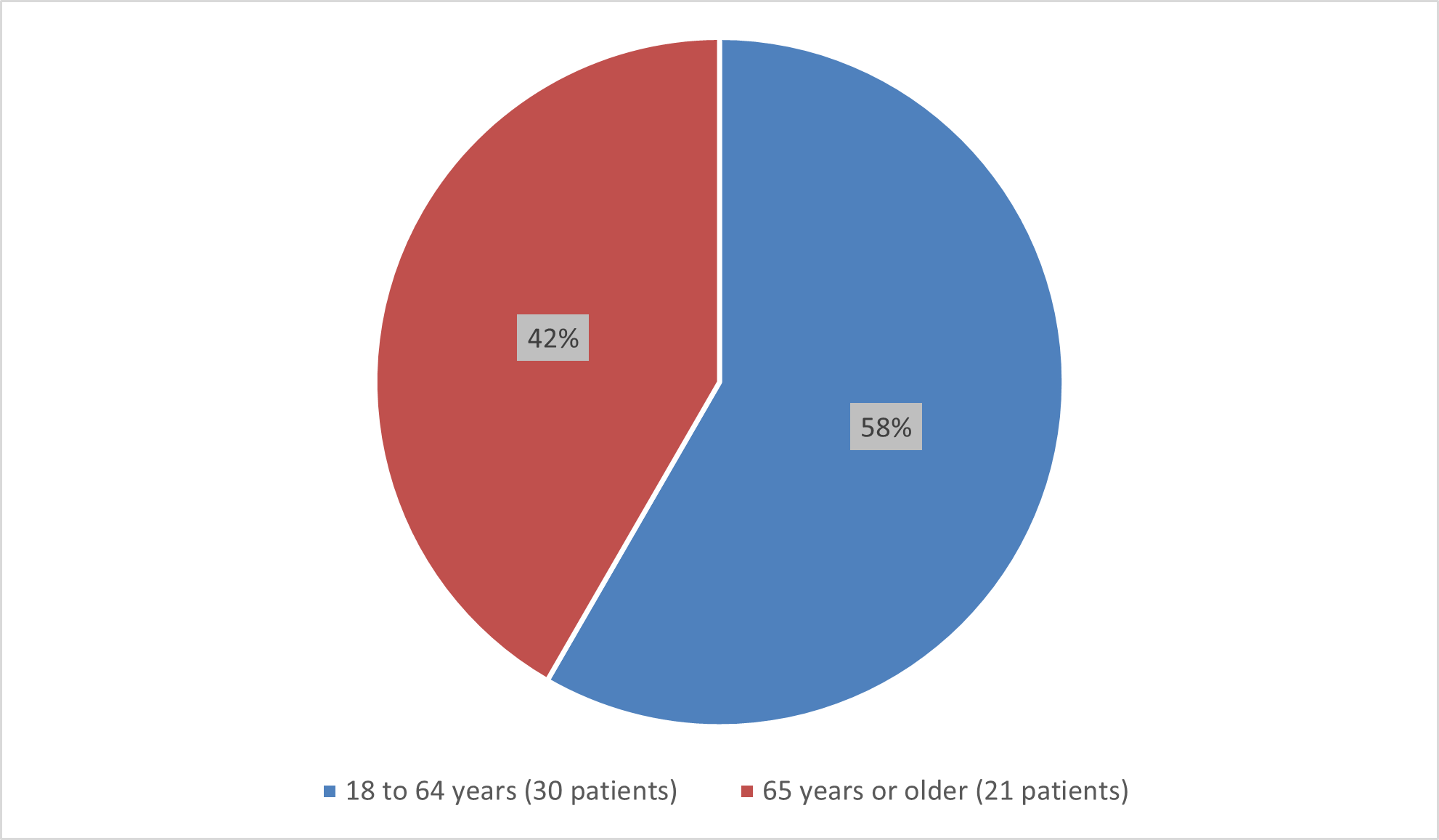
Figure 5 summarizes the percentage of patients by age group enrolled in the clinical trial used to evaluate the efficacy of BESREMi.
Figure 5. Baseline Demographics by Age (Efficacy Population)
Source: Adapted from FDA Review
Figure 6 summarizes the percentage of patients by age group enrolled in the combined trials used to evaluate the side effects of BESREMi.
Figure 6. Baseline Demographics by Age (Safety Population)
Source: Adapted from FDA Review
How were the trials designed?
BESREMi was evaluated in one clinical trial of 51 patients with polycythemia vera. In the trial, patients received the highest administered dose tolerated every two weeks subcutaneously. The starting dose was lower for patients who were transitioning from hydroxyurea.
The benefit of BESREMi was assessed by the proportion of patients who achieved normal blood counts, normal spleen size, and no new blood clots.
How were the trials designed?
The efficacy and safety of BESREMi were evaluated in the PEGINVERA study, a prospective, multicenter, single-arm trial of 7.5 years duration. All patients had the JAK2V617F mutation. The maximum tolerated dose was determined to be 540 µg. The efficacy of BESREMi was evaluated in the PEGINVERA study by assessing complete hematological response (CHR) defined as hematocrit <45% and no phlebotomy in the preceding 2 months, platelets ≤400 x109/L and leukocytes ≤10 x109/L, normal spleen size (longitudinal diameter ≤12 cm for females and ≤13 cm for males) assessed by ultrasound, and absence of thromboembolic events. The CHR in the treated population during the treatment period was 61% (31/51). The median duration of response was 14.3 months (95% CI: 5.5, 30.1). A hematological response based only on hematocrit, platelets, and leukocytes was achieved among 80% of patients treated with BESREMi (41/51). The median duration of this response was 20.8 months.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.