Drug Trials Snapshots: ELUCIREM
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the ELUCIREM Prescribing Information for all the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
ELUCIREM (gadopiclenol)
(ah – LOOS – er - em)
Guerbet LLC
Approval date: September 21, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ELUCIREM is a gadolinium-based contrast agent that is used during magnetic resonance imaging (MRI) in adult and pediatric patients aged 2 years and older to detect and visualize lesions with abnormal blood supply in the brain, spine, head and neck, thorax, abdomen, pelvis, and musculoskeletal system.
How is this drug used?
ELUCIREM is injected intravenously during an MRI scan.
Who participated in the clinical trials?
The FDA approved ELUCIREM based on evidence from eight clinical trials of 1,047 patients or healthy volunteers who received at least one dose of ELUCIREM, including two pivotal trials of ELUCIREM safety and effectiveness in 551 adults. Trial 1, which enrolled patients with central nervous system (CNS) lesions, was conducted at 33 sites in 11 countries and Trial 2, which enrolled patients with lesions outside the CNS, was conducted at 33 sites in 11 countries.
What are the benefits of this drug?
For patients getting an MRI, having images taken after ELUCIREM was given in addition to images taken before ELUCIREM was given helped make some abnormalities easier to see.
What are the benefits of this drug (results of trials used to assess efficacy)?
ELUCIREM was administered as an MRI contrast agent and its efficacy was established from two main studies: one for lesions in the CNS, and the other for lesions outside the CNS (termed body lesions). In each study, three independent, blinded readers evaluated the pre-contrast and paired (consisting of both pre-contrast and post-contrast images) images for three lesion visualization criteria (border delineation, internal morphology, and degree of contrast enhancement).
Table 1 summarizes the efficacy analysis results on patient-level average score of matching lesions for each visualization parameter between pre-contrast and paired image sets in the CNS lesion study (Study 1).
Table 1. Patient-Level CNS Lesion Visualization Scores by Reader, Paired vs. Pre Contrast in Patients Receiving ELUCIREM 0.05 mmol/kg Intravenously
| Parameter | N | LS Mean (SE) | 95% CI Difference | ||
|---|---|---|---|---|---|
| Paired | Pre-Contrast | Difference* | |||
| Border delineation | |||||
| Reader 1 | 227 | 3.90 (0.02) | 2.08 (0.02) | 1.82 (0.03) | 1.76, 1.88 |
| Reader 2 | 229 | 3.64 (0.04) | 1.74 (0.04) | 1.90 (0.05) | 1.81, 2.00 |
| Reader 3 | 202 | 3.97 (0.03) | 2.61 (0.03) | 1.36 (0.04) | 1.29, 1.44 |
| Internal morphology | |||||
| Reader 1 | 227 | 3.92 (0.03) | 1.66 (0.03) | 2.26 (0.03) | 2.20, 2.33 |
| Reader 2 | 229 | 3.65 (0.03) | 1.88 (0.03) | 1.77 (0.04) | 1.69, 1.85 |
| Reader 3 | 202 | 3.97 (0.04) | 2.01 (0.04) | 1.96 (0.05) | 1.85, 2.06 |
| Degree of contrast enhancement | |||||
| Reader 1 | 227 | 3.77 (0.03) | 1.00 (0.03) | 2.77 (0.04) | 2.69, 2.85 |
| Reader 2 | 229 | 3.58 (0.03) | 1.00 (0.03) | 2.58 (0.05) | 2.49, 2.67 |
| Reader 3 | 202 | 3.90 (0.02) | 1.00 (0.02) | 2.90 (0.03) | 2.84, 2.95 |
Source: ELUCIREM Prescribing Information
*p<0.0001 for all rows
Only matching lesions are considered. The mixed models based on the full analysis set (N=239) include lesion visualization factor as dependent variable, MRI modality (Pre-contrast and Paired MRI) as fixed factors, and patient as a random factor. Abbreviations: CI, confidence interval; CNS, central nervous system; LS, least squares; MRI, magnetic resonance imaging; SE, standard error
Table 2 summarizes the efficacy analysis results on patient-level average score of matching lesions for each visualization parameter between pre-contrast and paired image sets in the body lesion study (Study 2).
Table 2. Patient-Level Body Lesion Visualization Scores by Reader and Anatomic Region, Paired vs. Pre-Contrast in Patients Receiving ELUCIREM 0.05 mmol/kg Intravenously
| Parameter | n | LS Mean (SE) | 95% CI Difference |
||
|---|---|---|---|---|---|
| Paired | Pre-Contrast | Difference | |||
| Head and neck | |||||
| Border delineation | |||||
| Reader 1 | 15 | 3.71 (0.10) | 2.13 (0.10) | 1.58 (0.14) | 1.30, 1.86 |
| Reader 2 | 19 | 3.53 (0.18) | 2.11 (0.18) | 1.42 (0.18) | 1.06, 1.78 |
| Reader 3 | 13 | 3.92 (0.13) | 2.85 (0.13) | 1.08 (0.13) | 0.82, 1.33 |
| Internal morphology | |||||
| Reader 1 | 15 | 3.80 (0.07) | 1.87 (0.07) | 1.93 (0.10) | 1.74, 2.12 |
| Reader 2 | 19 | 3.74 (0.14) | 2.05 (0.14) | 1.68 (0.16) | 1.37, 2.00 |
| Reader 3 | 13 | 3.92 (0.12) | 2.54 (0.12) | 1.38 (0.14) | 1.10, 1.67 |
| Degree of contrast enhancement | |||||
| Reader 1 | 15 | 3.60 (0.11) | 1.00 (0.11) | 2.60 (0.16) | 2.29, 2.91 |
| Reader 2 | 19 | 3.68 (0.16) | 1.00 (0.16) | 2.68 (0.22) | 2.22, 3.15 |
| Reader 3 | 13 | 3.92 (0.11) | 1.00 (0.11) | 2.92 (0.15) | 2.61, 3.24 |
| Musculoskeletal system (including extremities) | |||||
| Border delineation | |||||
| Reader 1 | 17 | 3.00 (0.10) | 2.06 (0.10) | 0.94 (0.13) | 0.68, 1.20 |
| Reader 2 | 17 | 2.68 (0.20) | 2.44 (0.20) | 0.24 (0.19) | -0.15, 0.62 |
| Reader 3 | 21 | 2.81 (0.10) | 2.05 (0.10) | 0.76 (0.10) | 0.56, 0.96 |
| Internal morphology | |||||
| Reader 1 | 17 | 3.00 (0.07) | 2.00 (0.07) | 1.00 (0.09) | 0.82, 1.18 |
| Reader 2 | 17 | 3.94 (0.15) | 2.35 (0.15) | 1.59 (0.17) | 1.25, 1.92 |
| Reader 3 | 21 | 2.90 (0.09) | 2.05 (0.09) | 0.86 (0.11) | 0.64, 1.08 |
| Degree of contrast enhancement | |||||
| Reader 1 | 17 | 2.82 (0.10) | 1.00 (0.10) | 1.82 (0.15) | 1.53, 2.12 |
| Reader 2 | 17 | 3.33 (0.17) | 1.00 (0.17) | 2.33 (0.24) | 1.847, 2.82 |
| Reader 3 | 21 | 3.06 (0.08) | 1.00 (0.08) | 2.06 (0.12) | 1.82, 2.31 |
| Body (thorax, abdomen, pelvis) | |||||
| Border delineation | |||||
| Reader 1 | 219 | 3.86 (0.03) | 2.28 (0.03) | 1.57 (0.04) | 1.50, 1.64 |
| Reader 2 | 194 | 3.54 (0.06) | 3.15 (0.06) | 0.40 (0.06) | 0.29, 0.51 |
| Reader 3 | 228 | 3.53 (0.03) | 1.69 (0.03) | 1.84 (0.03) | 1.78, 1.90 |
| Internal morphology | |||||
| Reader 1 | 219 | 3.86 (0.02) | 2.00 (0.02) | 1.87 (0.03) | 1.82, 1.92 |
| Reader 2 | 194 | 3.74 (0.05) | 3.41 (0.05) | 0.33 (0.05) | 0.23, 0.43 |
| Reader 3 | 228 | 3.78 (0.03) | 1.60 (0.03) | 2.17 (0.03) | 2.11, 2.24 |
| Degree of contrast enhancement | |||||
| Reader 1 | 219 | 3.71 (0.03) | 1.00 (0.03) | 2.71 (0.04) | 2.63, 2.79 |
| Reader 2 | 194 | 2.69 (0.05) | 1.00 (0.05) | 1.69 (0.07) | 1.54, 1.83 |
| Reader 3 | 228 | 3.33 (0.03) | 1.00 (0.03) | 2.33 (0.44) | 2.25, 2.40 |
Source: ELUCIREM Prescribing Information
Only matching lesions are considered. The mixed models based on the full analysis set (N=278) include lesion visualization factor as a dependent variable, patient as a random factor, and MRI modality (Pre-contrast and Paired MRI), body regions, and MRI body regions as fixed factors.
Abbreviations: CI, confidence interval; LS, least squares; MRI, magnetic resonance imaging; SE, standard error
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: ELUCIREM worked similarly in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in how ELUCIREM worked among races could not be determined.
- Age: ELUCIREM worked similarly in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3 and Table 4 below summarize efficacy results by age. As shown, efficacy results were similar in patients younger and older than 65 years of age.
Table 3. Difference in Average Patient-Level Lesion Visualization Scores Between Paired and Pre-Contrast MRI for ELUCIREM by Patient Age in Study 1
| Parameter | Age <65 | Age ≥65 | ||||
|---|---|---|---|---|---|---|
| n | Difference | 95% CI | n | Difference | 95% CI | |
| Border delineation | ||||||
| Reader 1 | 143 | 1.85 | 1.77, 1.93 | 84 | 1.77 | 1.66, 1.87 |
| Reader 2 | 146 | 1.88 | 1.76, 2.00 | 83 | 1.94 | 1.78, 2.10 |
| Reader 3 | 126 | 1.40 | 1.30, 1.49 | 76 | 1.31 | 1.18, 1.43 |
| Internal morphology | ||||||
| Reader 1 | 143 | 2.27 | 2.18, 2.35 | 84 | 2.25 | 2.14, 2.36 |
| Reader 2 | 146 | 1.71 | 1.61, 1.81 | 83 | 1.87 | 1.74, 2.00 |
| Reader 3 | 126 | 1.98 | 1.85, 2.11 | 76 | 1.91 | 1.74, 2.08 |
| Degree of contrast enhancement | ||||||
| Reader 1 | 143 | 2.75 | 2.65, 2.85 | 84 | 2.81 | 2.68, 2.94 |
| Reader 2 | 146 | 2.54 | 2.42, 2.65 | 83 | 2.66 | 2.50, 2.81 |
| Reader 3 | 126 | 2.87 | 2.80, 2.94 | 76 | 2.94 | 2.86, 3.03 |
Source: GDX-44-010 Clinical Study Report, Figure 11-1
Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging
Table 4. Difference in Average Patient-Level Lesion Visualization Scores Between Paired and Pre-Contrast MRI for ELUCIREM by Patient Age in Study 2
| Parameter |
Age <65 |
Age ≥65 |
||||
| n | Difference | 95% CI | n | Difference | 95% CI | |
| Border delineation | ||||||
| Reader 1 | 164 | 1.56 | 1.48, 1.65 | 87 | 1.46 | 1.34, 1.58 |
| Reader 2 | 147 | 0.56 | 0.42, 0.69 | 83 | 0.32 | 0.14, 0.50 |
| Reader 3 | 176 | 1.73 | 1.65, 1.81 | 86 | 1.68 | 1.56, 1.81 |
| Internal morphology | ||||||
| Reader 1 | 164 | 1.85 | 1.79, 1.92 | 87 | 1.74 | 1.65, 1.83 |
| Reader 2 | 147 | 0.59 | 0.46, 0.73 | 83 | 0.42 | 0.24, 0.60 |
| Reader 3 | 176 | 2.04 | 1.95, 2.14 | 86 | 2.00 | 1.87, 2.14 |
| Degree of contrast enhancement | ||||||
| Reader 1 | 164 | 2.67 | 2.57, 2.77 | 87 | 2.59 | 2.46, 2.73 |
| Reader 2 | 147 | 1.93 | 1.75, 2.10 | 83 | 1.63 | 1.40, 1.86 |
| Reader 3 | 176 | 2.39 | 2.30, 2.47 | 86 | 2.23 | 2.10, 2.35 |
Source: GDX-44-011 Clinical Study Report, Figure 11-5
Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging
As shown in Table 5 and Table 6 below, efficacy results were similar between sexes.
Table 5. Difference in Average Patient-Level Lesion Visualization Scores Between Paired and Pre-Contrast MRI for ELUCIREM by Patient Sex in Study 1
| Parameter | Female | Male | ||||
| n | Difference | 95% CI | n | Difference | 95% CI | |
| Border delineation | ||||||
| Reader 1 | 119 | 1.79 | 1.70, 1.88 | 108 | 1.86 | 1.77, 1.95 |
| Reader 2 | 116 | 1.85 | 1.71, 1.98 | 113 | 1.96 | 1.82, 2.09 |
| Reader 3 | 99 | 1.34 | 1.23, 1.45 | 103 | 1.39 | 1.28, 1.49 |
| Internal morphology | ||||||
| Reader 1 | 119 | 2.22 | 2.13, 2.31 | 108 | 2.30 | 2.21, 2.40 |
| Reader 2 | 116 | 1.78 | 1.67, 1.89 | 113 | 1.76 | 1.64, 1.87 |
| Reader 3 | 99 | 1.94 | 1.79, 2.09 | 103 | 1.97 | 1.83, 2.12 |
| Degree of contrast enhancement | ||||||
| Reader 1 | 119 | 2.74 | 2.63, 2.85 | 108 | 2.81 | 2.69, 2.93 |
| Reader 2 | 116 | 2.56 | 2.43, 2.69 | 113 | 2.60 | 2.47, 2.73 |
| Reader 3 | 99 | 2.90 | 2.83, 2.98 | 103 | 2.89 | 2.82, 2.96 |
Source: GDX-44-010 Clinical Study Report, Figure 11-1
Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging
Table 6. Difference in Average Patient-Level Lesion Visualization Scores Between Paired and Pre-Contrast MRI for ELUCIREM by Patient Sex in Study 2
| Parameter | Female | Male | ||||
| n | Difference | 95% CI | n | Difference | 95% CI | |
| Border delineation | ||||||
| Reader 1 | 148 | 1.56 | 1.47, 1.65 | 103 | 1.49 | 1.38, 1.60 |
| Reader 2 | 130 | 0.55 | 0.40, 0.69 | 100 | 0.37 | 0.21, 0.54 |
| Reader 3 | 159 | 1.79 | 1.70, 1.88 | 103 | 1.60 | 1.49, 1.70 |
| Internal morphology | ||||||
| Reader 1 | 148 | 1.86 | 1.79, 1.93 | 103 | 1.75 | 1.66, 1.83 |
| Reader 2 | 130 | 0.54 | 0.39, 0.69 | 100 | 0.52 | 0.35, 0.69 |
| Reader 3 | 159 | 2.08 | 1.98, 2.18 | 103 | 1.95 | 1.83, 2.08 |
| Degree of contrast enhancement | ||||||
| Reader 1 | 148 | 2.69 | 2.58, 2.79 | 103 | 2.57 | 2.45, 2.70 |
| Reader 2 | 130 | 1.88 | 1.70, 2.07 | 100 | 1.74 | 1.53, 1.95 |
| Reader 3 | 159 | 2.36 | 2.27, 2.45 | 103 | 2.30 | 2.18, 2.41 |
Source: GDX-44-011 Clinical Study Report, Figure 11-5
Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging
Table 7 and Table 8 below summarize efficacy results by race.
Table 7. Difference in Average Patient-Level Lesion Visualization Scores Between Paired and Pre-Contrast MRI for ELUCIREM by Patient Race in Study 1
| Parameter | White | Asian | ||||
|---|---|---|---|---|---|---|
| n | Difference | 95% CI | n | Difference | 95% CI | |
| Border delineation | ||||||
| Reader 1 | 190 | 1.83 | 1.77, 1.90 | 17 | 1.59 | 1.36, 1.82 |
| Reader 2 | 190 | 1.91 | 1.81, 2.02 | 18 | 1.89 | 1.55, 2.23 |
| Reader 3 | 173 | 1.37 | 1.29, 1.45 | 15 | 1.07 | 0.79, 1.34 |
| Internal morphology | ||||||
| Reader 1 | 190 | 2.27 | 2.20, 2.34 | 17 | 2.18 | 1.94, 2.42 |
| Reader 2 | 190 | 1.77 | 1.68, 1.86 | 18 | 1.94 | 1.65, 2.23 |
| Reader 3 | 173 | 1.99 | 1.88, 2.10 | 15 | 1.40 | 1.03, 1.77 |
| Degree of contrast enhancement | ||||||
| Reader 1 | 190 | 2.75 | 2.66, 2.84 | 17 | 3.00 | 2.70, 3.30 |
| Reader 2 | 190 | 2.56 | 2.46, 2.67 | 18 | 2.72 | 2.39, 3.06 |
| Reader 3 | 173 | 2.89 | 2.83, 2.95 | 15 | 3.00 | 2.81, 3.19 |
| Parameter | Black or African American | American Indian or Alaska Native | ||||
| n | Difference | 95% CI | n | Difference | 95% CI | |
| Border delineation | ||||||
| Reader 1 | 3 | 1.67 | 1.12, 2.22 | 15 | 1.93 | 1.69, 2.18 |
| Reader 2 | 4 | 1.50 | 0.78, 2.2 | 15 | 1.97 | 1.59, 2.34 |
| Reader 3 | 0 | 13 | 1.62 | 1.32, 1.91 | ||
| Internal morphology | ||||||
| Reader 1 | 3 | 2.00 | 1.43, 2.57 | 15 | 2.30 | 2.04, 2.56 |
| Reader 2 | 4 | 1.50 | 0.88, 2.12 | 15 | 1.60 | 1.28, 1.92 |
| Reader 3 | 0 | 13 | 2.15 | 1.76, 2.55 | ||
| Degree of contrast enhancement | ||||||
| Reader 1 | 3 | 3.00 | 2.29, 3.71 | 15 | 2.73 | 2.42, 3.05 |
| Reader 2 | 4 | 2.50 | 1.79, 3.21 | 15 | 2.60 | 2.23, 2.97 |
| Reader 3 | 0 | 13 | 2.85 | 2.64, 3.05 | ||
| Parameter | Native Hawaiian or Other Pacific Islander | Other | ||||
| n | Difference | 95% CI | n | Difference | 95% CI | |
| Border delineation | ||||||
| Reader 1 | 1 | 2.00 | 1 | 1.83 | 1.77, 1.90 | |
| Reader 2 | 1 | 2.00 | 1 | 1.91 | 1.81, 2.02 | |
| Reader 3 | 0 | 1 | 1.37 | 1.29, 1.45 | ||
| Internal morphology | ||||||
| Reader 1 | 1 | 2.00 | 1 | 2.27 | 2.20, 2.34 | |
| Reader 2 | 1 | 2.00 | 1 | 1.77 | 1.68, 1.86 | |
| Reader 3 | 0 | 1 | 1.99 | 1.88, 2.10 | ||
| Degree of contrast enhancement | ||||||
| Reader 1 | 1 | 3.00 | 1 | 2.75 | 2.66, 2.84 | |
| Reader 2 | 1 | 3.00 | 1 | 2.56 | 2.46, 2.67 | |
| Reader 3 | 0 | 1 | 2.89 | 2.83, 2.95 | ||
Source: GDX-44-010 Clinical Study Report, Figure 11-1 and Table 14.2.1.16
Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging
Table 8. Difference in Average Patient-Level Lesion Visualization Scores Between Paired and Pre-Contrast MRI for ELUCIREM by Patient Race in Study 2
| Parameter | White | Asian | ||||
|---|---|---|---|---|---|---|
| n | Difference | 95% CI | n | Difference | 95% CI | |
| Border delineation | ||||||
| Reader 1 | 179 | 1.53 | 1.44, 1.61 | 40 | 1.50 | 1.32, 1.68 |
| Reader 2 | 164 | 0.39 | 0.27, 0.52 | 36 | 0.57 | 0.29, 0.84 |
| Reader 3 | 186 | 1.68 | 1.59, 1.76 | 41 | 1.78 | 1.60, 1.95 |
| Internal morphology | ||||||
| Reader 1 | 179 | 1.81 | 1.75, 1.88 | 40 | 1.81 | 1.68, 1.95 |
| Reader 2 | 164 | 0.45 | 0.32, 0.57 | 36 | 0.63 | 0.35, 0.90 |
| Reader 3 | 186 | 1.99 | 1.90, 2.08 | 41 | 2.17 | 1.97, 2.36 |
| Degree of contrast enhancement | ||||||
| Reader 1 | 179 | 2.64 | 2.54, 2.74 | 40 | 2.56 | 2.35, 2.76 |
| Reader 2 | 164 | 1.86 | 1.69, 2.02 | 36 | 1.63 | 1.27, 1.98 |
| Reader 3 | 186 | 2.32 | 2.24, 2.41 | 41 | 2.24 | 2.08, 2.43 |
| Parameter | Black or African American | American Indian or Alaska Native | ||||
| n | Difference | 95% CI | n | Difference | 95% CI | |
| Border delineation | ||||||
| Reader 1 | 6 | 1.67 | 1.20, 2.13 | 24 | 1.57 | 1.34, 1.80 |
| Reader 2 | 4 | 0.13 | -0.70, 0.95 | 24 | 0.91 | 0.57, 1.25 |
| Reader 3 | 6 | 2.00 | 1.54, 2.46 | 27 | 1.80 | 1.59, 2.02 |
| Internal morphology | ||||||
| Reader 1 |
6 |
2.00 | 1.65, 2.35 | 24 | 1.75 | 1.58, 1.92 |
| Reader 2 | 4 | 0.50 | -0.33, 1.33 | 24 | 0.99 | 0.66, 1.33 |
| Reader 3 | 6 | 2.17 | 1.65, 2.68 | 27 | 2.07 | 1.83, 2.32 |
| Degree of contrast enhancement | ||||||
| Reader 1 | 6 | 3.00 | 2.47, 3.53 | 24 | 2.67 | 2.40, 2.93 |
| Reader 2 | 4 | 1.75 | 0.69, 2.81 | 24 | 1.85 | 1.42, 2.29 |
| Reader 3 | 6 | 2.67 | 2.19, 3.14 | 27 | 2.47 | 2.25, 2.69 |
| Parameter | Other | |||||
| n | Difference | 95% CI | ||||
| Border delineation | ||||||
| Reader 1 | 2 | 1.50 | 0.70, 2.30 | |||
| Reader 2 | 2 | 0.50 | -0.67, 1.67 | |||
| Reader 3 | 2 | 2.00 | 1.21, 2.79 | |||
| Internal morphology | ||||||
| Reader 1 | 2 | 2.00 | 1.39, 2.61 | |||
| Reader 2 | 2 | 0.50 | -0.67, 1.67 | |||
| Reader 3 | 2 | 2.00 | 1.10, 2.90 | |||
| Degree of contrast enhancement | ||||||
| Reader 1 | 2 | 3.00 | 2.09, 3.91 | |||
| Reader 2 | 2 | 2.00 | 0.51, 3.49 | |||
| Reader 3 | 2 | 2.50 | 1.68, 3.32 | |||
Source: GDX-44-011 Clinical Study Report, Figure 11-5
Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging
What are the possible side effects?
The most common side effects were injection site discomfort (pain, warmth, or coldness), headache, nausea, dizziness, and local swelling.
What are the possible side effects (results of trials used to assess safety)?
The safety of ELUCIREM was evaluated in 1,047 patients who received ELUCIREM at doses ranging from 0.025 mmol/kg (one half the recommended dose) to 0.3 mmol/kg (six times the recommended dose). A total of 708 patients received the recommended dose of 0.05 mmol/kg. Overall, approximately 4.7% of subjects receiving the labeled dose reported one or more adverse reactions.
Table 7. Adverse Reactions That Occurred in >0.2% of Patients Who Received ELUCIREM 0.05 mmol/kg
| Adverse Reaction | ELUCIREM 0.05 mmol/kg N=708 % |
|---|---|
| Injection site pain | 0.7 |
| Headache | 0.7 |
| Nausea | 0.4 |
| Injection site warmth | 0.4 |
| Injection site coldness | 0.3 |
| Dizziness | 0.3 |
| Local swelling | 0.3 |
Source: ELUCIREM Prescribing Information
Adverse reactions that occurred with a frequency ≤0.2% in patients who received 0.05 mmol/kg ELUCIREM included: maculopapular rash, vomiting, worsened renal impairment, feeling hot, pyrexia, oral paresthesia, dysgeusia, diarrhea, pruritus, allergic dermatitis, erythema, injection site paresthesia, cystatin C increase, and blood creatinine increase.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was slightly higher in females.
- Race: The number of patients of races other than White was small; therefore, differences in side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
There was a mildly greater incidence of adverse events (AEs) in females than would be expected based on enrollment. Notable differences between sexes at the system organ class level included general disorders and administration site conditions (7.1% of females versus 3.8% of males) and nervous system disorders (6.1% of females versus 2.9% of males, largely driven by increased headache in females).
Table 8. Adverse Events by Patient Sex Among Patients Who Received ELUCIREM at the Recommended Dose
| Sex | Patients N=708 n (%) |
Patients With AE N=119 n (%) |
Number of AE N=188 n (%) |
|---|---|---|---|
| Female | 393 (56) | 75 (63) | 130 (69) |
| Male | 315 (44) | 44 (37) | 58 (31) |
Source: Pooled Safety Data from gadopiclenol clinical studies, Table 41
Abbreviations: AE, adverse event
The fractions of pediatric patients and of older adult patients who experienced at least one adverse event were similar to or mildly lower than the fractions of those age groups enrolled in the clinical studies.
Table 9. Adverse Events by Patient Age Among Patients Who Received ELUCIREM at the Recommended Dose
| Age Group, Years | Patients N=708 n (%) |
Patients With AE N=119 n (%) |
Number of AE N=188 n (%) |
|---|---|---|---|
| 2 to 17 | 80 (11%) | 14 (12%) | 31 (17%) |
| 18 to 64 | 424 (60%) | 82 (69%) | 121 (64%) |
| ≥65 | 204 (29%) | 23 (19%) | 36 (19%) |
| ≥75 | 55 (8%) | 4 (3%) | 4 (2%) |
Source: Pooled Safety Data from gadopiclenol clinical studies, Table 35
Abbreviations: AE, adverse event
DEMOGRAPHICS SNAPSHOT
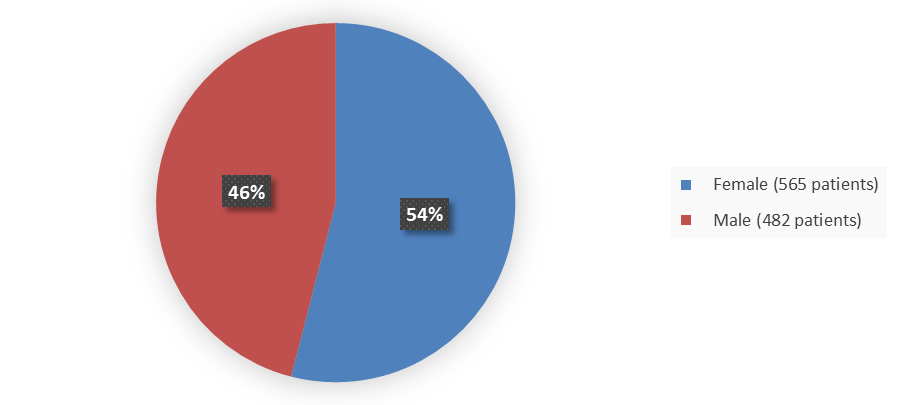
Figure 1 summarizes how many male and female patients were enrolled in the combined clinical trials.
Figure 1. Baseline Demographics by Sex (Safety Population)
Source: Adapted from FDA Review
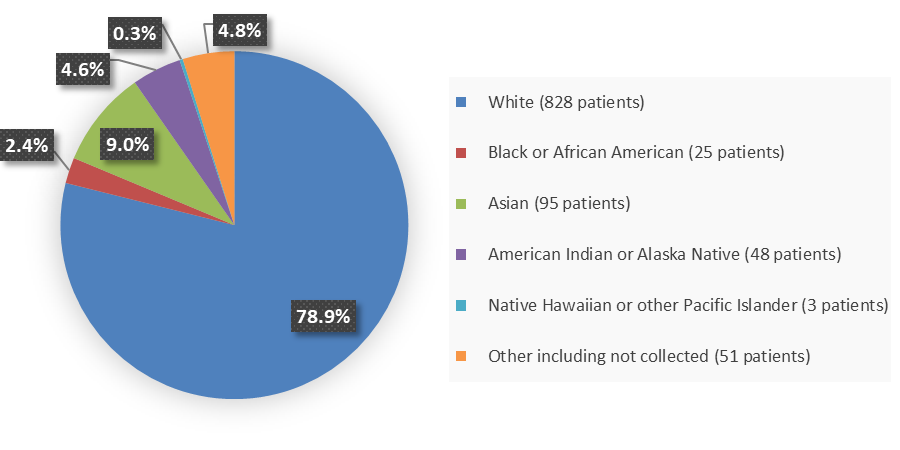
Figure 2 summarizes the percentage of patients by race enrolled in the combined clinical trials.
Figure 2. Baseline Demographics by Race (Safety Population)
Source: Adapted from FDA Review
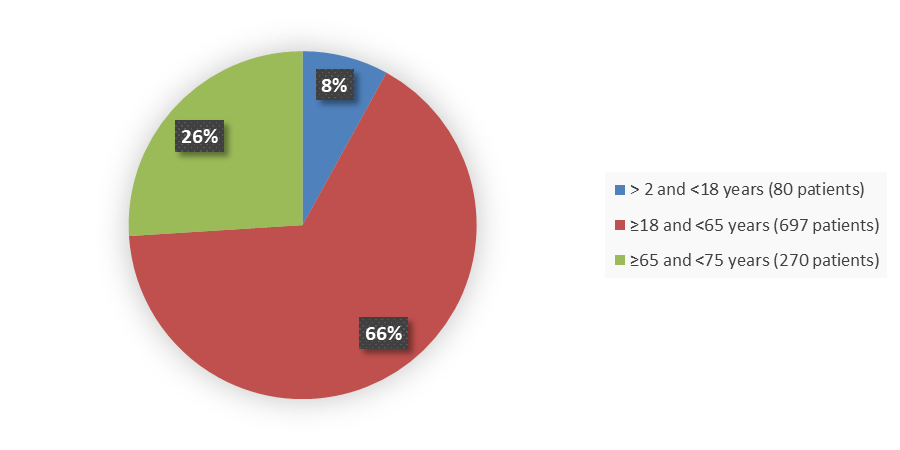
Figure 3 summarizes the percentage of patients by age group in the combined clinical trials.
Figure 3. Baseline Demographics by Age (Safety Population)
Source: Adapted from FDA Review
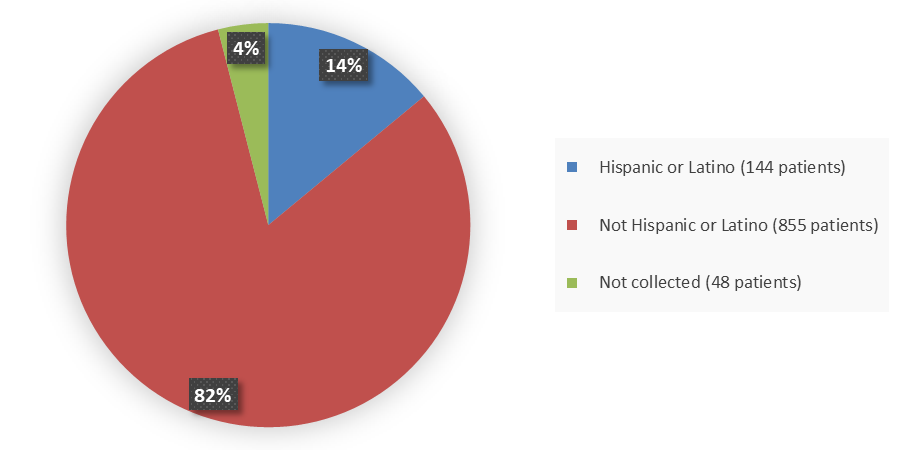
Figure 4 summarizes the percentage of patients by ethnicity in the combined clinical trials.
Figure 4. Baseline Demographics by Ethnicity (Safety Population)
Source: Adapted from FDA Review
Who participated in the trials?
Table 10. Demographic and Baseline Characteristics of the Pooled Safety Sets
| Parameter | Pooled 0.05 mmol/kg Safety Set N=708 n (%) |
Pooled Safety Set N=1047 n (%) |
|---|---|---|
| Age, years | ||
| Mean (SD) | 51.2 (19.9) | 50.8 (18.4) |
| Median | 55.0 | 55.0 |
| Min, max | 2, 88 | 2, 88 |
| Age group, years | ||
| ≤17 | 80 (11) | 80 (8) |
| <65 | 504 (71) | 777 (74) |
| ≥65 | 204 (29) | 270 (26) |
| ≥75 | 55 (8) | 62 (6) |
| Sex | ||
| Female | 393 (56) | 565 (54) |
| Male | 315 (44) | 482 (46) |
| Race | ||
| White | 563 (80) | 828 (79) |
| Black or African American | 15 (2) | 25 (2) |
| Asian | 71 (10) | 95 (9) |
| American Indian or Alaska Native | 47 (7) | 48 (5) |
| Native Hawaiian or Other Pacific Islander | 3 (<1) | 3 (<1) |
| Other | 3 (<1) | 3 (<1) |
| Not collected | 9 (1) | 48 (5) |
| Ethnicity | ||
| Hispanic or Latino | 91 (13) | 144 (14) |
| Not Hispanic or Latino | 608 (86) | 855 (82) |
| Not collected | 9 (1) | 48 (4) |
| Region | ||
| United States | 104 (15) | 134 (13) |
| Outside United States | 604 (85) | 913 (87) |
| Region | ||
| North America | 173 (25) | 255 (25) |
| Europe | 469 (66) | 705 (67) |
| Asia | 66 (9) | 87 (8) |
| Dose, mmol/kg | ||
| Mean (SD) | 0.051 (0.005) | 0.078 (0.064) |
| Min, max | 0.025, 0.104 | 0.023, 0.307 |
| Renal impairment at baseline | ||
| Moderate (eGFR <60 mL/min/1.73 m2 and ≥30 mL/min/1.73 m2) | 46 (6) | 57 (5) |
| Severe (eGFR <30 mL/min/1.73 m2 or on dialysis) | 0 | 16 (2) |
| Other characteristics at baseline | ||
| Hepatic insufficiency | 40 (6) | 67 (6) |
| Cardiac disease | 72 (10) | 100 (10) |
| History of allergic diseases | 96 (14) | 120 (12) |
Source: Adapted from FDA Review
Note: For patients who received more than one dose of gadopiclenol, only the highest dose per patient was used for calculating the average in the Pooled Safety Set. A single dose of gadopiclenol was administered to patients in the Pooled 0.05 mmol/kg Safety Set.
Abbreviations: eGFR, estimated glomerular filtration rate; max, maximum; min, minimum; SD, standard deviation
How were the trials designed?
The benefits of ELUCIREM were mainly evaluated in two confirmatory studies in 551 adults who received a single dose each. Study 1 was performed in adults with abnormalities in the brain or spinal cord. Study 2 was performed in adults with abnormalities outside the brain or spinal cord.
In each study, images of the affected body part were obtained using MRI both before and after patients received a dose of ELUCIREM. Three radiologists scored the images based on how well the abnormalities were seen. The benefit of ELUCIREM was evaluated by comparing the scores from the combined images obtained before and after ELUCIREM to the scores from the images obtained before ELUCIREM.
How were the trials designed?
Study 1 was performed in adults with known or highly suspected CNS lesions with focal areas of disruption of the blood-brain barrier. Study 2 was performed in adults with suspected enhancing abnormalities in at least one body region among the head and neck, thorax, abdomen, pelvis, and musculoskeletal system.
In each study, patients received both ELUCIREM 0.05 mmol/kg and gadobutrol 0.1 mmol/kg (as an active comparator) in random order separated by 2 to 14 days. Magnetic resonance imaging was performed before and after administration of each contrast agent.
Pre-contrast and paired (consisting of both pre-contrast and post-contrast images for the same drug) image sets were independently evaluated by three central readers who were blinded to the identity of the contrast agent. Readers scored up to three lesions per patient for border delineation, internal morphology, and contrast enhancement, each on a scale from 1 to 4. The total number of lesions was also reported. The analysis compared the patient-level average score for matching lesions for each visualization parameter between pre-contrast and paired image sets.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.