Drug Trials Snapshots: ROLVEDON
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the ROLVEDON Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
ROLVEDON (eflapegrastim)
(Roll veh don)
SPECTRUM PHARMACEUTICALS, INC.
Approval date: September 9, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ROLVEDON is a drug used to stimulate the growth of neutrophils, a type of white blood cell important in the body’s fight against infection in patients with cancers that do not involve bone marrow (non-myeloid cancers) who receive certain anti-cancer medicines that can suppress the bone marrow from producing blood cells and cause fever and low neutrophil count.
How is this drug used?
ROLVEDON is an injection given under the skin (subcutaneously) approximately 24 hours after administration of certain anticancer medicine (chemotherapy) that is toxic to cells.
Who participated in the clinical trials?
The FDA approved ROLVEDON based on evidence from two clinical trials of 643 patients with breast cancer treated with anti-cancer drugs that suppress the bone marrow from producing blood cells. The trials were conducted at 119 sites in 6 countries in the United States, Canada, South-Korea, Hungary, Poland, and India.
How were the trials designed?
ROLVEDON was evaluated in two clinical trials of 643 patients with breast cancer receiving anticancer treatment that is known to suppress the growth of blood-forming cells (red blood cells, white blood cells, and platelets) in the bone marrow. In both trials, patients were randomly assigned to either receive ROLVEDON or pegfilgrastim under the skin (subcutaneously) approximately 24 hours after anticancer treatment. Patients in both groups were evaluated and compared for the duration of severe neutropenia (a condition with lower-than-normal levels of neutrophils in the blood) during the first cycle of anticancer therapy.
How were the trials designed?
The safety and efficacy evaluation of ROLVEDON to decrease the incidence of infection, as manifested by febrile neutropenia, in patients with non-myeloid malignancies receiving myelosuppressive anticancer drugs associated with clinically significant incidence of febrile neutropenia was based on the results of two clinical trials.
Both trials were randomized, open-label, active-controlled, non-inferiority trials of similar design. Patients were treated with four cycles of myelosuppressive chemotherapy. Patients were randomized (1:1) and received a fixed dose of ROLVEDON (13.2 mg/0.6 mL) or pegfilgrastim (6 mg/0.6 mL) subcutaneously on Day 2 of each cycle after chemotherapy. The primary endpoint in both trials was the comparison of the duration of severe neutropenia (absolute neutrophil count <0.5×109/L) in Cycle 1.
DEMOGRAPHICS SNAPSHOT
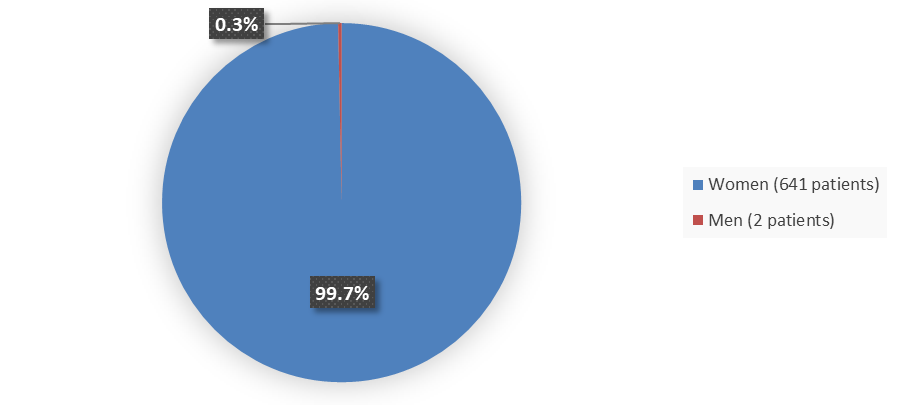
Figure 1. Baseline Demographics by Sex (Efficacy Population)
Source: Adapted from FDA Review
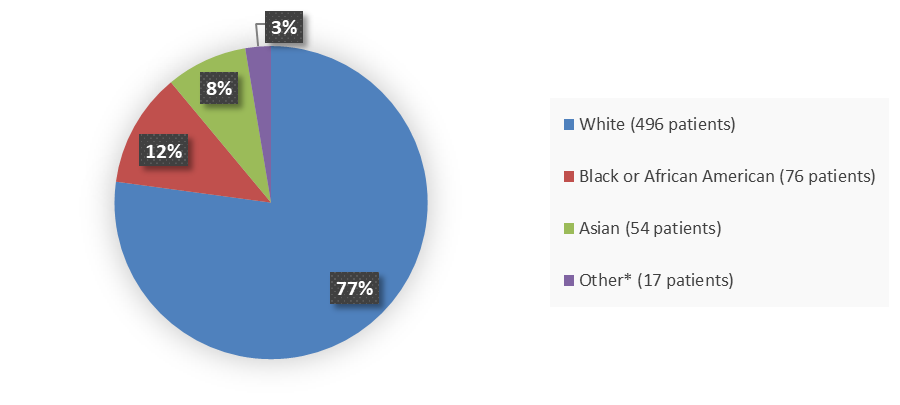
Figure 2. Baseline Demographics by Race (Efficacy Population)
Source: Adapted from FDA Review
* Other includes 3 (<1%) American Indian or Alaska Native, 1 (<1%) Native Hawaiian or other Pacific Islander, and 13 (2%) patients with no race data reported.
Table 1. Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 496 | 77 |
| Black or African American | 76 | 12 |
| Asian | 54 | 8 |
| Other | 17 | 3 |
| Not reported | 13 | 2 |
| American Indian or Alaska Native | 3 | <1 |
| Native Hawaiian or Other Pacific Islander | 1 | <1 |
Source: Adapted from FDA Review
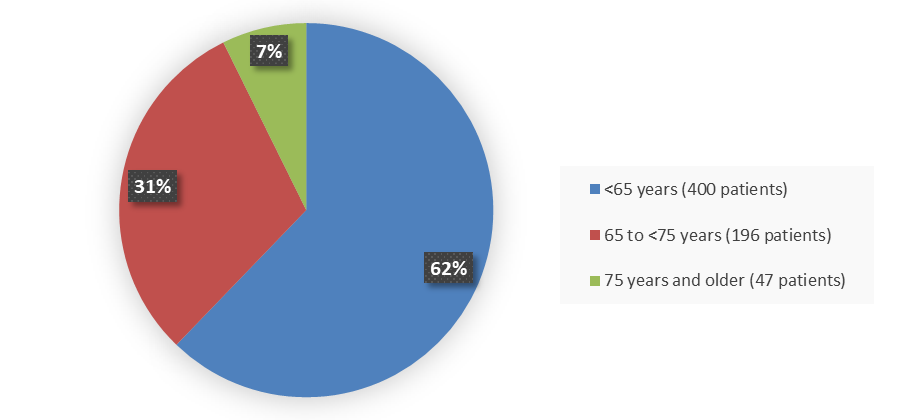
Figure 3. Baseline Demographics by Age (Efficacy Population)
Source: Adapted from FDA Review
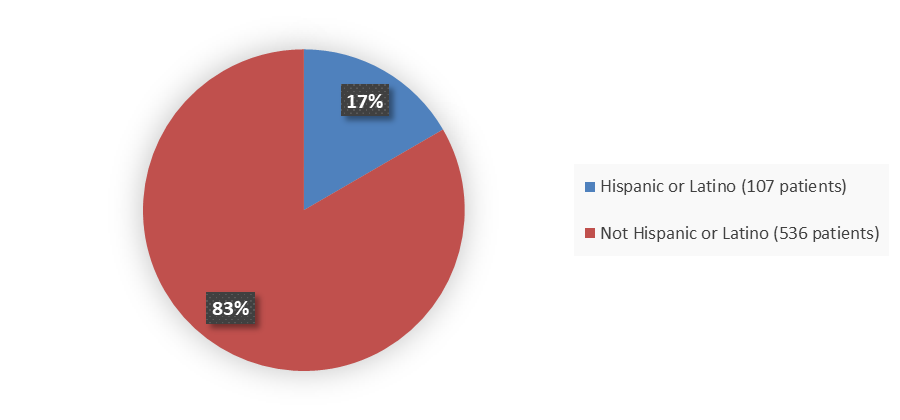
Figure 4. Baseline Demographics by Ethnicity (Efficacy Population)
Source: Adapted from FDA Review
Who participated in the trials?
Table 2. summarizes demographics of patients that participated in both trials.
Table 2. Trial Demographics (Trials 1 and 2 Combined)
| Demographic Parameter | ROLVEDON N=314 n (%) |
Pegfilgrastim N=329 n (%) |
Total N=643 n (%) |
|---|---|---|---|
| Sex | |||
| Female | 313 (99.7) | 328 (99.7) | 641 (99.7) |
| Male | 1 (0.3) | 1 (0.3) | 2 (0.3) |
| Race | |||
| White | 241 (76.8) | 255 (77.5) | 496 (77.1) |
| Black or African American | 37 (11.8) | 39 (11.9) | 76 (11.8) |
| Asian | 29 (9.2) | 25 (7.6) | 54 (8.4) |
| Other | 7 (2.2) | 10 (3.0) | 17 (2.6) |
| Age, years | |||
| Mean (SD) | 59.2 (11.2) | 58.7 (12.1) | 58.9 (11.7) |
| Median | 60 | 60 | 60 |
| Range | 28 - 83 | 24 - 88 | 24 - 88 |
| Age group, years | |||
| <65 | 192 (61.1) | 208 (63.2) | 400 (62.2) |
| ≥65 to <75 | 104 (33.1) | 92 (28.0) | 196 (30.5) |
| ≥75 | 18 (5.7) | 29 (8.8) | 47 (7.3) |
| Ethnicity | |||
| Hispanic or Latino | 52 (16.6) | 55 (16.7) | 107 (16.6) |
| Not Hispanic or Latino | 262 (83.4) | 274 (83.3) | 536 (83.4) |
| Region | |||
| North America | 261 (83.1) | 275 (83.6) | 536 (83.4) |
| United States only | 252 (80.3) | 272 (82.7) | 524 (81.5) |
| Central Europe | 35 (11.1) | 36 (10.9) | 71 (11.0) |
| Asia | 18 (5.7) | 18 (5.5) | 36 (5.6) |
Source: Adapted from FDA Review
Abbreviations: SD, standard deviation
What are the benefits of this drug?
In patients receiving certain (myelosuppressive) anticancer treatment, ROLVEDON relative to pegfilgrastim was similar in decreasing the duration of severe neutropenia (a condition with abnormally low count of neutrophils) in Cycle 1 in two similarly designed clinical trials. Pegfilgrastim, like ROLVEDON is a drug approved to stimulate the growth of neutrophils.
What are the benefits of this drug (results of trials used to assess efficacy)?
Efficacy results from the two trials are presented in Table 1. The primary endpoint was the duration of severe neutropenia in Cycle 1.
Table 3. Duration of Severe Neutropenia in Cycle 1 (Study 1 and Study 2)
| Parameter | Study 1 | Study 2 | ||
|---|---|---|---|---|
| ROLVEDON N=196 |
Pegfilgrastim N=210 |
ROLVEDON N=118 |
Pegfilgrastim N=119 |
|
| Mean DSN (SD), days | 0.20 (0.503) | 0.35 (0.683) | 0.31 (0.688) | 0.39 (0.949) |
| Median DSN (range), days | 0 (0, 3) | 0 (0, 3) | 0 (0, 3) | 0 (0, 7) |
| Difference in DSN, days | -0.148 | -0.073 | ||
| *95% confidence intervala | -0.265, -0.033 | -0.292, 0.129 | ||
Source: ROLVEDON Prescribing Information
a Confidence intervals were obtained using 2.5 percentile and 97.5 percentile of the 100,000 bootstrap samples with treatment as stratification factor.
* The non-inferiority of ROLVEDON to pegfilgrastim was to be declared if the upper bound of 95% CI of the difference in mean DSN between the treatment arms was <0.62 days.
Abbreviations: CI, confidence interval; DSN, duration of severe neutropenia; SD, standard deviation
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: The number of males was small; therefore, differences in how ROLVEDON worked between males and females could not be determined.
- Race: The effect of ROLVEDON was similar in White, Black/African-American, Asian and Other races of patients.
- Age: The effect of ROLVEDON relative to pegfilgrastim was similar in patients younger than 65 years of age and 65 years of age and above.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 4 presents analyses of primary endpoint by demographic subgroups.
Table 4. Subgroup Analyses of Duration of Severe Neutropenia in Cycle 1
| Parameter | Study 1 Difference With Pegfilgrastim (95% CI) |
Study 2 Difference With Pegfilgrastim (95% CI) |
|---|---|---|
| Age, years | ||
| <65 | -0.112 (-0.253, 0.029) | -0.113 (-0.370, 0.145) |
| ≥65 | -0.212 (-0.415, -0.009) | -0.023 (-0.397, 0.352) |
| Race | ||
| White | -0.128 (-0.255, -0.002) | -0.047 (-0.296, 0.202) |
| Non-White | -0.212 (-0.509, 0.086) | -0.175 (-0.599, 0.249) |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval
What are the possible side effects?
ROLVEDON may cause serious side effects including:
- Splenic rupture
- A serious lung problem called acute respiratory distress syndrome
- Serious allergic reactions
- Sickle cell crisis in patients with sickle cell disorders
- Kidney injury (glomerulonephritis)
- Increased white blood cell count (leukocytosis)
- Decreased platelet count (thrombocytopenia)
- Fluid leak from blood vessels into the body’s tissues (capillary leak syndrome)
- Potential for tumor growth stimulatory effects on malignant cells
- Myelodysplastic syndrome and acute myeloid leukemia in patients with breast and lung cancer
- Inflammation of the aorta (aortitis)
The most common side effects are fatigue, nausea, diarrhea, bone pain, headache, fever, anemia, rash, myalgia, arthralgia, and back pain.
What are the possible side effects (results of trials used to assess safety)?
Table 5 summarizes adverse reactions that occurred in the clinical trials.
Table 5. Adverse Reactions Occurring in ≥10% of Patients With Breast Cancer Who Received ROLVEDON (Safety Population)
| Adverse Reaction | ROLVEDON N=314 n (%) |
Pegfilgrastim N=326 n (%) |
|---|---|---|
| Fatigue | 181 (58) | 192 (59) |
| Nausea | 162 (52) | 166 (51) |
| Diarrhea | 125 (40) | 126 (39) |
| Bone pain | 119 (38) | 121 (37) |
| Headache | 92 (29) | 90 (28) |
| Pyrexia | 87 (28) | 84 (26) |
| Anemia | 77 (25) | 52 (16) |
| Rash | 77 (25) | 99 (30) |
| Myalgia | 69 (22) | 49 (15) |
| Arthralgia | 66 (21) | 48 (15) |
| Back pain | 63 (20) | 55 (17) |
| Decreased appetite | 61 (19) | 50 (15) |
| Edema peripheral | 57 (18) | 53 (16) |
| Abdominal pain | 53 (17) | 67 (21) |
| Dizziness | 50 (16) | 38 (12) |
| Dyspnea | 49 (16) | 44 (13) |
| Cough | 48 (15) | 51 (16) |
| Thrombocytopenia | 44 (14) | 17 (5) |
| Pain | 37 (12) | 42 (13) |
| Pain in extremity | 36 (11) | 42 (13) |
| Local administration reactions* | 34 (11) | 27 (8) |
| Flushing | 32 (10) | 27 (8) |
Source: ROLVEDON Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The number of males was small; therefore, differences in side effects of ROLVEDON between males and females could not be determined.
- Race: The occurrence of side effects was similar in White,, Black/African-American, Asian and Other races of patients.
- Age: The occurrence of side effects was similar in patients younger than 65 years of age and 65 years of age and above.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 6 summarize adverse reactions by subgroups noted in the clinical trials.
Table 6. Overview of Side Effects by Sex, Race, and Age in Trials SPI-GCF-301 and SPI-GCF-302, Safety Population
| Rolvedon N=314 |
Pegfilfrastim N=326 |
|||||
|---|---|---|---|---|---|---|
| Characteristic | All Patients n (%) |
All Grades n/Ns (%) |
Grades 3 to 4 n/Ns (%) |
All Patients n (%) |
All Grades n/Ns (%) |
Grades 3 to 4 n/Ns (%) |
| Sex, n (%) | ||||||
| Female | 313 (99.7) | 306/313 (97.8) | 236/313 (75.4) | 325 (99.7) | 319/325 (98.2) | 236/325 (72.6) |
| Male | 1 (0.3) | 1/1 (100) | 1/1 (100) | 1 (0.3) | 1/1 (100) | 1/1 (100) |
| Race, n (%) | ||||||
| White | 241 (76.8) | 236/241 (97.9) | 185/241 (76.8) | 252 (77.3) | 247/252 (98.0) | 187/252 (74.2) |
| Black or African American | 37 (11.8) | 35/37 (94.6) | 23/37 (62.2) | 39 (12.0) | 39/39 (100) | 26/39 (66.7) |
| Asian | 29 (9.2) | 29/29 (100) | 25/29 (86.2) | 25 (7.7) | 24/25 (96.0) | 16/25 (64.0) |
| American Indian or Alaska Native | 2 (0.6) | 2/2 (100) | 1/2 (50.0) | 1 (0.3) | 1/1 (100) | 0/1 (0) |
| Native Hawaiian or Other Pacific Islander | 0 (0) | 0/0 (NA) | 0/0 (NA) | 1 (0.3) | 1/1 (100) | 1/1 (100) |
| Other | 5 (1.6) | 5/5 (100) | 3/5 (60.0) | 8 (2.5) | 8/8 (100) | 7/8 (87.5) |
| Age group, years, n (%) | ||||||
| <65 | 192 (61.1) | 186/192 (96.9) | 134/192 (69.8) | 205 (62.9) | 201/205 (98.0) | 145/205 (70.7) |
| ≥65 | 122 (38.9) | 121/122 (99.2) | 103/122 (84.4) | 121 (37.1) | 119/121 (98.3) | 92/121 (76.0) |
Source: adae.xpt (eCTD seq 0000); FDA reviewer's analysis
Abbreviation: N, number of patients in the safety population; n, number of patients with given characteristic; Ns, total number of patients in each category
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.