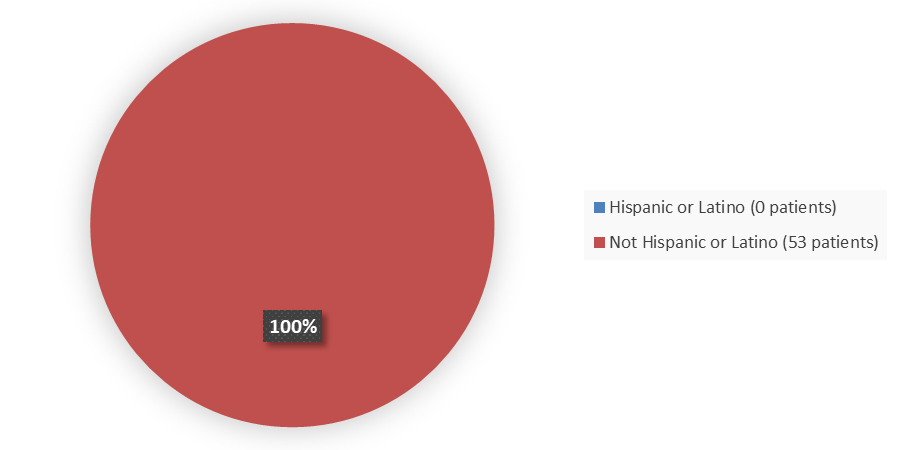Drug Trials Snapshots: SPEVIGO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the SPEVIGO Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
SPEVIGO (spesolimab-sbzo)
(speh-VEE-goh)
Boehringer Ingelheim Pharmaceuticals, Inc.
Approval date: September 1, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
SPEVIGO is an interleukin-36 receptor (IL-36R) antagonist that is approved for the treatment of generalized pustular psoriasis (GPP) flare in adults.
How is this drug used?
SPEVIGO is given in the vein (intravenous) once over 90 minutes. If flare symptoms persist, a second single dose may be administered one week after the first dose.
Who participated in the clinical trials?
The FDA approved SPEVIGO based on evidence from a clinical trial of 53 adult patients with generalized pustular psoriasis flare. The trial was conducted at 3 sites in the United States and 23 sites globally (Africa, Asia, and Europe).
How were the trials designed?
SPEVIGO was evaluated in one clinical trial (Study Effisayil-1/NCT03782792) of 53 adult patients with GPP flare. In the trial, patients received a single treatment with either SPEVIGO or placebo. Patients were evaluated for clearance of pustules based on a Generalized Pustular Psoriasis Physician Global Assessment (GPPPGA) pustulation sub score of 0 (indicating no visible pustules) at Week 1. Neither the patient nor the healthcare providers knew which treatment was being given until after Week 1. After Week 1, all patients, whether they initially received SPEVIGO or placebo, who continued to experience flare symptoms, had the option to receive a single open-label treatment of SPEVIGO (second treatment and first treatment for patients in the SPEVIGO and placebo groups, respectively). After Week 1 to Week 12, patients in either original treatment group whose GPP flare reoccurred after achieving a clinical response were eligible to receive a single open-label rescue treatment of SPEVIGO, with a maximum of 3 total treatments of SPEVIGO throughout the trial.
How were the trials designed?
A randomized, double-blind, placebo-controlled study (Study Effisayil-1/NCT03782792) was conducted to evaluate the clinical efficacy and safety of SPEVIGO in adult patients with flares of GPP. Patients were randomized if they had a flare of GPP of moderate-to-severe intensity, as defined by:
- A GPPPGA total score of at least 3 (moderate) on a scale from 0 (clear) to 4 (severe),
- The presence of fresh pustules (new appearance or worsening of pustules),
- GPPPGA pustulation sub score of at least 2 (mild), and
- At least 5% of body surface area covered with erythema and the presence of pustules.
Patients were required to discontinue systemic and topical therapy for GPP prior to receiving study drug.
A total of 53 patients were randomized (2:1) to receive a single intravenous dose of 900 mg SPEVIGO (N=35) or placebo (N=18) administered over 90 minutes during the double-blind portion of the study.
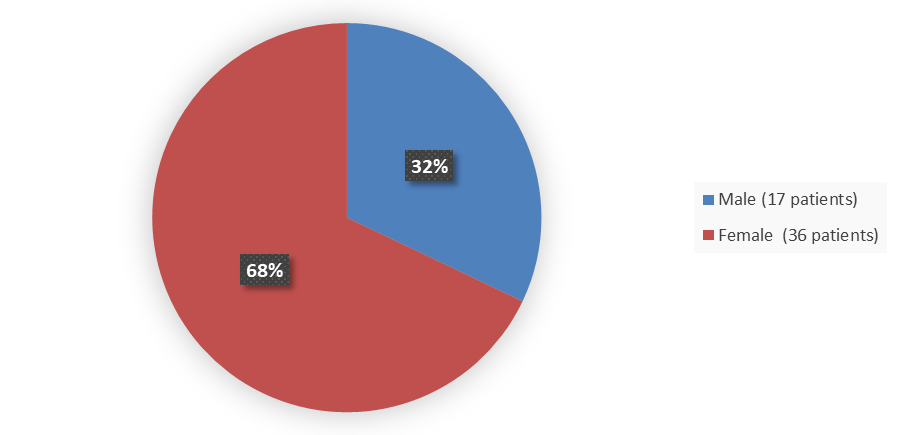
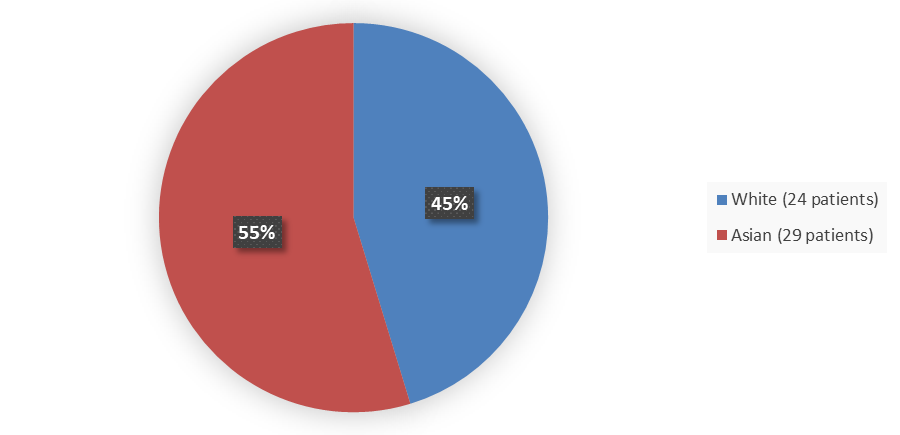
The study population consisted of 32% males and 68% females. The mean age was 43 years (range: 21 to 69 years); 55% of patients were Asian and 45% were White. Most patients included in the study had a GPPPGA pustulation sub score of 3 (43%) or 4 (36%), and patients had a GPPPGA total score of 3 (81%) or 4 (19%). In this study, 25% of patients had been previously treated with biologic therapy for GPP.
The primary endpoint of the study was the proportion of patients with a GPPPGA pustulation sub score of 0 (indicating no visible pustules) at Week 1 after treatment.
Patients in either treatment group who continued to experience flare symptoms at Week 1 were eligible to receive a single open-label intravenous dose of 900 mg of SPEVIGO (second dose for patients in the SPEVIGO and first dose for patients in the placebo groups). At Week 1, 12 (34%) patients in the SPEVIGO group and 15 (83%) patients in the placebo group received open-label SPEVIGO. After Week 1 to Week 12, patients in either treatment group whose GPP flare reoccurred after achieving a clinical response were eligible to receive a single open-label rescue intravenous dose of 900 mg of SPEVIGO, with a maximum of 3 total doses of SPEVIGO throughout the study. Six patients received a single open-label rescue dose of SPEVIGO. Thirty-six patients received 1 dose of SPEVIGO, 13 patients received 2 doses of SPEVIGO, and 2 patients received 3 doses of SPEVIGO throughout the study.
DEMOGRAPHICS SNAPSHOT
Figure 1. Baseline Demographics by Sex (Efficacy Population)
Source: Adapted from FDA Review
Figure 2. Baseline Demographics by Race (Efficacy Population)
Source: Adapted from FDA Review
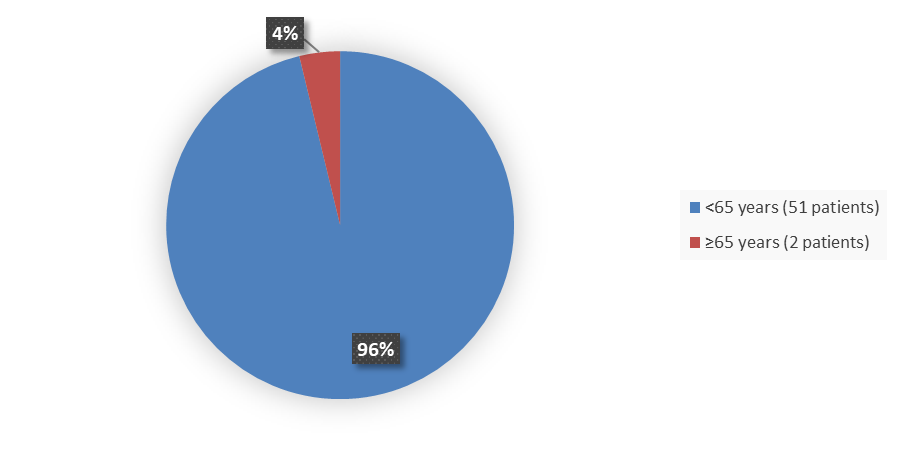
Figure 3. Baseline Demographics by Age (Efficacy Population)
Source: Adapted from FDA Review
Figure 4. Baseline Demographics by Ethnicity (Efficacy Population)
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. summarizes demographics for the randomized population in the clinical trial.
Table 1. Demographic Characteristics for Patients in Study Effisayil-1
| Subgroup | SPEVIGO N=35 n (%) |
Placebo N=18 n (%) |
Total N=53 n (%) |
|---|---|---|---|
| Sex | |||
| Female | 21 (60) | 15 (83) | 36 (68) |
| Male | 14 (40) | 3 (17) | 17 (32) |
| Age, years | |||
| Mean | 43.2 | 42.6 | 43 |
| Standard deviation | 12.1 | 8.4 | 10.9 |
| Minimum | 21 | 30 | 21 |
| Median | 41 | 41.5 | 41 |
| Maximum | 69 | 57 | 69 |
| Age group, years | |||
| <65 | 33 (94) | 18 (100) | 51 (96) |
| ≥65 | 2 (6) | 0 (0) | 2 (4) |
| Race | |||
| Asian | 16 (46) | 13 (72) | 29 (55) |
| White | 19 (54) | 5 (28) | 24 (45) |
| Ethnicity | |||
| Not Hispanic or Latino | 35 (100) | 18 (100) | 53 (100) |
| Region | |||
| Africa | 5 (14) | 2 (11) | 7 (13) |
| Asia | 14 (40) | 13 (72) | 27 (51) |
| Europe | 14 (40) | 2 (11) | 16 (30) |
| United States | 2 (6) | 1 (6) | 3 (6) |
Source: Adapted from FDA review
What are the benefits of this drug?
More patients achieved clearance in pustules after treatment with SPEVIGO in comparison to those treated with placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
In the pivotal clinical efficacy trial (Study Effisayil-1) with 53 adult patients with a GPP flare, 35 patients received SPEVIGO and 18 patients received placebo. Nineteen of the 35 patients who received SPEVIGO (54%) compared to 1/18 patients who received placebo (6%) had clearance of pustules at Week 1.
Table summarizes efficacy results for the clinical trial based on the primary efficacy endpoint defined as the proportion of patients with a GPPPGA pustulation sub score of 0 (indicating no visible pustules) at Week 1 after treatment.
Table 2. GPPPGA Pustulation Sub Score at Week 1 in Study Effisayil-1
| Endpoint | SPEVIGO N=35 |
Placebo N=18 |
|---|---|---|
| Subjects achieving a GPPPGA pustulation sub score of 0, n (%) | 19 (54) | 1 (6) |
| Risk difference versus placebo, % (95% CI) | 49 (21, 67) | |
Source: SPEVIGO Prescribing Information
Abbreviations: CI, confidence interval; GPPPGA, Generalized Pustular Psoriasis Physician Global Assessment
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: The observed effect of SPEVIGO was similar for females and males.
- Race: The observed effect of SPEVIGO was similar for White and Asian participants. All patients were Asian or White; therefore, differences in how SPEVIGO worked among other races could not be determined.
- Age: The number of patients 65 years of age or older was small; therefore, differences in how SPEVIGO worked across age groups could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3 summarizes efficacy results by age, sex, and race in Study Effisayil-1.
Table 3. Results of the Primary Efficacy Endpoint by Age, Sex, and Race in Study Effisayil-1 (RS1)
| Demographic | SPEVIGO N=35 n/Ns (%) |
Placebo N=18 n/Ns (%) |
Difference (95% CI2) |
|---|---|---|---|
| Age, years | |||
| <65 | 18/33 (55) | 1/18 (6) | 49 (21, 68) |
| ≥65 | 2/2 (100) | - | - |
| Sex | |||
| Male | 8/14 (57) | 0/3 (0) | 57 (-19, 82) |
| Female | 11/21 (52) | 1/15 (7) | 46 (15, 69) |
| Race | |||
| Asian | 10/16 (63) | 1/13 (8) | 55 (17, 80) |
| White | 9/19 (47) | 0/5 (0) | 47 (-7, 72) |
Source: Adapted from FDA Review
1 Randomized Set (RS): all randomized subjects. Missing data were imputed using non-responder imputation. Subjects who received escape medication, open-label SPEVIGO at Day 8, or rescue medication with SPEVIGO were imputed as non-responders.
2 Confidence interval based on the Wilson method.
Abbreviations: CI, confidence interval; n, number of patients with primary efficacy endpoint; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
What are the possible side effects?
SPEVIGO may cause serious side effects including infection, allergic reactions, and infusion-related reactions.
The most common side effects include fatigue, nausea and vomiting, headache, itching or itchy bumps, bruising, and urinary tract infection.
What are the possible side effects (results of trials used to assess safety)?
The most frequent side effect that occurred in patients treated with SPEVIGO in the trial were infections and were reported in 14% of patients treated with SPEVIGO compared to 6% of patients treated with placebo during the 1-week placebo-controlled period.
Two cases of drug reaction with eosinophilia and systemic symptoms were reported in the trial in patients treated with SPEVIGO. Guillain-Barre syndrome was reported in patients treated with SPEVIGO.
Injection site reactions were also reported in patients treated with SPEVIGO during clinical development.
Table 4 summarizes adverse reactions that occurred in the clinical trial.
Table 4. Selected Adverse Reactions Occurring in ≥1% of the SPEVIGO Group and More Frequently Than in the Placebo Group Through Week 1 (Study Effisayil-1)
| Adverse Reaction | SPEVIGO N=35 n (%) |
Placebo N=18 n (%) |
|---|---|---|
| Asthenia and fatigue | 3 (9) | 0 |
| Nausea and vomiting | 3 (9) | 1 (6) |
| Headache | 3 (9) | 1 (6) |
| Pruritus and prurigo | 2 (6) | 0 |
| Infusion site hematoma and bruising | 2 (6) | 0 |
| Urinary tract infection | 2 (6) | 0 |
| Bacteremia | 1 (3) | 0 |
| Bacteriuria | 1 (3) | 0 |
| Cellulitis | 1 (3) | 0 |
| Herpes dermatitis and oral herpes | 1 (3) | 0 |
| Upper respiratory tract infection | 1 (3) | 0 |
| Dyspnea | 1 (3) | 0 |
| Eye edema | 1 (3) | 0 |
| Urticaria | 1 (3) | 0 |
Source: SPEVIGO Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The trial did not include enough patients to determine if there are differences in the occurrence of side effects according to biological sex.
- Race: The trial did not include enough patients to determine if there are differences in the occurrence of side effects according to race.
- Age: The trial did not include enough patients to determine if there are differences in the occurrence of side effects according to age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Due to the rarity of the disease, the trial did not include sufficient numbers of patients to determine if there are differences in side effects according to biological sex, race, and age.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.