Drug Trials Snapshot: COSELA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the COSELA Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
COSELA (trilaciclib)
koh-sel-uh
G1 Therapeutics, Inc.
Approval date: February 12, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
COSELA is a prescription medicine used to decrease the rate of bone marrow suppression (myelosuppression) resulting from chemotherapy in adult patients when given prior to certain chemotherapy drugs (containing platinum/etoposide or topotecan) for a type of lung cancer (extensive stage-small cell lung cancer) that is widespread.
How is this drug used?
COSELA is given into the vein through an intravenous (IV) line before each dose of certain chemotherapy drugs.
What are the benefits of this drug?
COSELA reduces the duration of bone marrow suppression (severe neutropenia) in adult patients with extensive stage-small cell lung cancer receiving certain types of chemotherapy.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table (Table 1) below summarizes the results of the primary and the secondary efficacy endpoints for Study 1.
Table 1: Study 1: Myeloprotective Efficacy Results in Patients Treated with COSELA or Placebo Prior to Chemotherapy (Intent-to-Treat Analysis
|
Endpoint |
COSELA |
Placebo |
Treatment Effecta |
Adjusted |
|---|---|---|---|---|
|
Primary Endpoints |
||||
|
DSN in Cycle 1 - days |
0 (1.0) |
4 (4.7) |
-3.6* |
<0.0001 |
|
Number (%) of patients with severe neutropenia |
1 (1.9%) |
26 (49.1%) |
0.038 |
<0.0001 |
|
Key Secondary Endpoints |
||||
|
Number of all-cause dose reductions, event rate per cycle |
0.021 |
0.085 |
0.242 |
0.0195 |
|
Number (%) of patients with RBC transfusion on/after 5 weeks |
7 (13.0%) |
11 (20.8%) |
0.642 |
-- |
|
Number (%) of patients with G-CSF administration |
16 (29.6%) |
25 (47.2%) |
0.646 |
-- |
ANCOVA=analysis of covariance; CI=confidence interval; DSN= Duration of Severe Neutropenia; G CSF=granulocyte colony-stimulating factor; N=total number of patients in each treatment group; RBC=red blood cells; SD=standard deviation; SN=severe neutropenia.
a The following statistical models were used to assess treatment effects: non-parametric ANCOVA (DSN in Cycle 1); modified Poisson regression (occurrence of SN, and RBC transfusion on/after 5 weeks); negative binomial regression (number of all-cause dose reductions). All models included the following as covariates: ECOG status, presence of brain metastases, and the corresponding baseline laboratory values.
b One-sided adjusted p-value obtained from a Hochberg-based gatekeeping procedure. Source: COSELA Prescribing Information, Section 14, Table 5
The table below (Table 2) summarizes the results of the primary and the secondary efficacy endpoints for Study 2.
Table 2: Study 2: Myeloprotective Efficacy Results in Patients Treated with COSELA or Placebo Prior to Chemotherapy (Intent-to-Treat Analysis)
|
Endpoint |
COSELA |
Placebo |
|---|---|---|
|
DSN in Cycle 1 (days): mean (SD) |
0 (0.5) |
3 (3.9) |
|
Number (%) of patients with severe neutropenia |
2 (5.1%) |
16 (42.1%) |
|
Number of all-cause dose reductions, event rate per cycle |
0.022 |
0.084 |
|
Number (%) of patients with RBC transfusion on/after 5 weeks |
2 (5.1%) |
9 (23.7%) |
|
Number (%) of patients with G-CSF administration |
4 (10.3%) |
24 (63.2%) |
DSN=duration of severe neutropenia; G-CSF=granulocyte colony-stimulating factor;
N=total number of patients in each treatment group; RBC=red blood cell; SD=standard deviation
Source: COSELA Prescribing Information, Section 14, Table 6
The table below (Table 3) summarizes the results of the primary and the secondary efficacy endpoints for Study 3.
Table 3: Myeloprotective Efficacy Results in Patients Treated with COSELA or Placebo Prior to Chemotherapy (Intent-to-Treat Analysis)
|
Endpoint |
COSELA |
Placebo |
|---|---|---|
|
Primary Endpoints |
||
|
DSN in Cycle 1 (days): mean (SD) |
2 (3.9) |
7 (6.2) |
|
Number (%) of patients with severe neutropenia |
13 (40.6%) |
22 (75.9%) |
|
Key Secondary Endpoints |
||
|
Number of all-cause dose reductions, event rate per cycle |
0.051 |
0.116 |
|
Number (%) of patients with RBC transfusion on/after 5 weeks |
10 (31.3%) |
12 (41.4%) |
|
Number (%) of patients with G-CSF administration |
16 (50.0%) |
19 (65.5%) |
|
Number (%) of patients with platelet transfusion |
8 (25.0%) |
9 (31.0%) |
DSN=duration of severe neutropenia; G-CSF=granulocyte colony-stimulating factor;
N=total number of patients in each treatment group; RBC=red blood cell; SD=standard deviation
Source: COSELA Prescribing Information, Section 14, Table 6
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: COSELA appeared to be similarly effective in men and women.
- Race: The number of patients of races other than White was small. Therefore, differences among races in how well the COSELA worked could not be determined.
- Age: COSELA appeared to be similarly effective in patients < 65 years and ≥ 65 years.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Subgroup efficacy results by sex, race and age were only provided on Study 1, since Study 1 was the pre-planned pivotal study, and Study 2 and Study 3 were considered as supportive studies with small sample sizes.
Table 4: Primary Endpoint Analysis on Occurrence of Severe Neutropenia by Sex, Race and Age (ITT Analysis Set) for Study 1
|
Occurrence of Severe |
COSELA |
Placebo |
|---|---|---|
|
Sex |
||
|
Male |
2.6 (1/39) |
35.3 (12/34) |
|
Female |
0 (0/13) |
63.2 (12/19) |
|
Race |
||
|
White |
2.0 (1/51) |
47.1 (24/51) |
|
Black or African American |
0 |
0 (0/1) |
|
Native Hawaiian or Other Pacific Islander |
0 (0/1) |
0 |
|
Other |
0 |
0 (0/1) |
|
Age |
||
|
<65 |
3.7 (1/27) |
33.3 (9/27) |
|
>=65 |
0 (0/25) |
57.7 (15/26) |
FDA’s Analysis
What are the possible side effects?
The most common side effects included feeling tired, low levels of calcium, potassium, and phosphorus in the blood, increase in an enzyme produced by the liver, headache, and pneumonia. Other less common side effects include allergic reactions that may be serious (anaphylaxis), reactions at the site of injection, and inflammation of the lungs.
What are the possible side effects (results of trials used to assess safety)?
Table 5: Adverse Reactions in ≥5% Patients with SCLC Receiving COSELA (with ≥2% Higher Incidence in COSELA Compared to Placebo)
|
Adverse Reaction |
COSELA (N=122) |
Placebo (N=118) |
||
|---|---|---|---|---|
|
All Gradesa |
Grade ≥3 |
All Gradesa |
Grade ≥3 |
|
|
Fatigue |
34 |
3 |
27 |
2 |
|
Hypocalcemiab |
24 |
<1 |
21 |
<1 |
|
Hypokalemiac |
22 |
6 |
18 |
3 |
|
Hypophosphatemiad |
21 |
7 |
16 |
2 |
|
Aspartate aminotransferasee |
17 |
<1 |
14 |
<1 |
|
Headache |
13 |
0 |
9 |
0 |
|
Pneumonia |
10 |
7 |
8 |
7 |
|
Rash |
9 |
<1 |
6 |
0 |
|
Infusion-related reaction |
8 |
0 |
2 |
0 |
|
Edema peripheral |
7 |
0 |
4 |
<1 |
|
Abdominal pain upper |
7 |
0 |
3 |
0 |
|
Thrombosis |
7 |
3 |
2 |
2 |
|
Hyperglycemia |
6 |
2 |
3 |
0 |
a Graded per NCI CTCAE v4.03x
b Hypocalcemia=calcium decreased (lab) or treatment-emergent adverse event (TEAE) preferred term 'Hypocalcemia'
c Hypokalemia=potassium decreased (lab) or TEAE preferred terms 'Hypokalemia,' 'Blood potassium decreased'
d Hypophosphatemia=phosphate decreased (lab) or TEAE preferred terms 'Hypophosphatemia,' 'Blood phosphorus decreased'
e Aspartate aminotransferase increased=aspartate aminotransferase increased (lab) or TEAE preferred term 'Blood aspartate aminotransferase increased'
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: The occurrence of side effects was generally similar in men and women
- Race: The number of patients of races other than White was small. Therefore, differences among races in how well the COSELA worked could not be determined.
- Age: The occurrence of side effects was generally similar in patients below and above 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The safety of COSELA was evaluated in Studies 1, 2, and 3 among 240 patients (122 patients in the COSELA group and 118 patients in the placebo group) who were being treated for extensive stage small cell lung cancer.
Table 6. Adverse Reactions by Sex
|
Adverse Reactions n, (%) |
COSELA |
Placebo |
||
|---|---|---|---|---|
|
Male |
Female |
Male |
Female |
|
|
Fatigue |
27 (31%) |
14 (40%) |
18 (25%) |
14 (30%) |
|
Hypocalcemia |
18 (21%) |
11 (31%) |
15 (21%) |
10 (22%) |
|
Hypokalemia |
18 (21%) |
9 (26%) |
10 (14%) |
11 (24%) |
|
Hypophosphatemia |
19 (22%) |
7 (20%) |
10 (14%) |
9 (20%) |
|
Aspartate Aminotransferase Increased |
12 (14%) |
9 (26%) |
10 (14%) |
6 (13%) |
|
Headache |
8 (9%) |
8 (23%) |
4 (6%) |
7 (15%) |
|
Pneumonia |
7 (8%) |
5 (14%) |
4 (6%) |
5 (11%) |
Table 7. Adverse Reactions by Race
|
Adverse Reactions n, (%) |
COSELA |
Placebo |
||
|---|---|---|---|---|
|
White |
Non-white |
White |
Non-white |
|
|
Fatigue |
40 (34%) |
1 (33%) |
28 (26%) |
4 (44%) |
|
Hypocalcemia |
27 (23%) |
2 (66%) |
24 (22%) |
1 (11%) |
|
Hypokalemia |
26 (22%) |
1 (33%) |
17 (16%) |
4 (44%) |
|
Hypophosphatemia |
25 (21%) |
1 (33%) |
15 (14%) |
4 (44%) |
|
Aspartate Aminotransferase Increased |
21 (18%) |
0 (0%) |
14 (13%) |
2 (22%) |
|
Headache |
15 (13%) |
1 (33%) |
10 (9%) |
1 (11%) |
|
Pneumonia |
11 (9%) |
1 (33%) |
9 (8%) |
0 (0%) |
Table 8. Adverse Reactions by Age
|
Adverse Reactions n, (%)
|
COSELA |
Placebo |
||
|---|---|---|---|---|
|
<65 years |
≥ 65 years |
<65 years |
≥65 years |
|
|
Fatigue |
18 (27%) |
23 (41%) |
14 (23%) |
18 (32%) |
|
Hypocalcemia |
12 (18%) |
17 (30%) |
13 (21%) |
12 (21%) |
|
Hypokalemia |
17 (26%) |
10 (18%) |
10 (16%) |
11 (19%) |
|
Hypophosphatemia |
12 *18%) |
14 (25%) |
7 (11%) |
12 (21%) |
|
Aspartate Aminotransferase Increased |
16 (24%) |
5 (9%) |
9 (15%) |
7 (12%) |
|
Headache |
10 (15%) |
6 (11%) |
7 (11%) |
4 (7%) |
|
Pneumonia |
8 (12%) |
4 (7%) |
5 (8%) |
4 (7%) |
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved COSELA based on evidence from 1 pivotal clinical trial (Study 1) of 107 patients with newly diagnosed extensive stage-small cell lung cancer receiving chemotherapy for the first time. The trial was conducted at 37 sites in 8 countries in USA, Ukraine, Latvia, Georgia, France, Estonia, Spain and Bulgaria. In addition, there were 2 supportive clinical trials. Study 2 enrolled 77 patients with extensive stage-small cell lung cancer receiving chemotherapy for the first time and Study 3 enrolled 61 patients with extensive stage-small cell lung cancer previously treated with chemotherapy.
Figure 1 summarizes how many men and women were enrolled in the combined clinical trials used to evaluate the efficacy of COSELA.
Figure 1. Baseline Demographics by Sex (ITT Population)
FDA’s Analysis
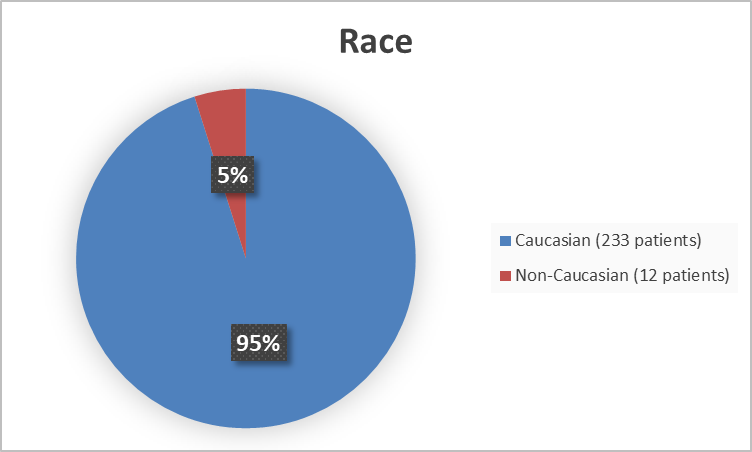
Figure 2 summarizes the percentage of patients by race enrolled in the combined clinical trials used to evaluate the efficacy of COSELA.
Figure 2. Baseline Demographics by Race
FDA’s Analysis
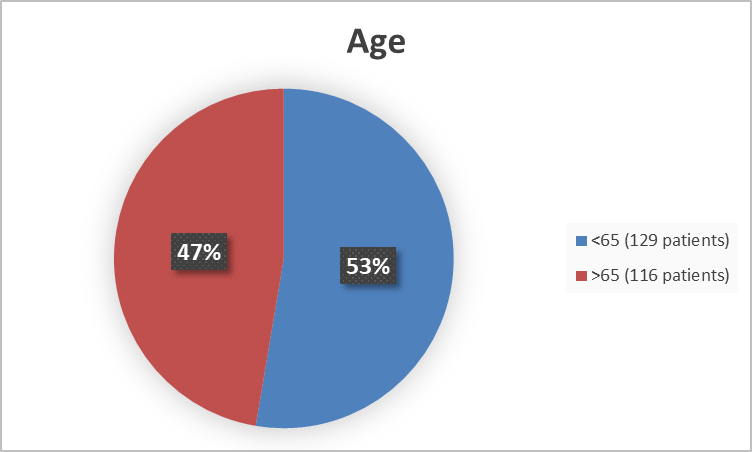
Figure 3 summarizes the percentage of patients by age enrolled in the combined clinical trials used to evaluate the efficacy of COSELA
Figure 3. Baseline Demographics by Age
FDA’s Analysis
Who participated in the trials?
Table 9 below summarizes demographics from 3 clinical trials by randomized treatment
Table 9: Demographics of Randomized Patients from 3 Clinical Trials
|
|
COSELA |
Placebo |
Total |
|---|---|---|---|
|
Sex |
|||
|
Male |
90 (55%) |
73 (45%) |
163 |
|
Female |
35 (43%) |
47 (57%) |
82 |
|
Race |
|||
|
Caucasian |
122 (52%) |
111 (48%) |
233 |
|
Non-Caucasian |
3 (25%) |
9 (75%) |
12 |
|
Age group |
|||
|
<65 |
67 (52%) |
62 (48%) |
129 |
|
>=65 |
58 (50%) |
58 (50%) |
116 |
FDA’s Analysis
How were the trials designed?
Three trials were conducted in adult patients with extensive stage-small cell lung cancer. In all three trials, patients were randomly assigned to receive COSELA or placebo prior to receiving chemotherapy. All three studies measured the duration of bone marrow suppression (severe neutropenia) and the number of patients with severe neutropenia in both the COSELA and placebo groups.
How were the trials designed?
The efficacy of COSELA was established in three randomized, double-blind, placebo-controlled trials in patients with extensive stage-small cell lung cancer (ES-SCLC). Study 1 enrolled adult patients receiving carboplatin, etoposide, and atezolizumab for newly diagnosed ES-SCLC. Study 2 enrolled adult patients receiving etoposide/carboplatin for newly diagnosed ES-SCLC. Study 3 enrolled adult patients receiving topotecan for previously treated ES-SCLC.
During Cycle 1, all three studies prohibited primary prophylactic granulocyte-colony stimulating factor (G-CSF) and erythropoiesis-stimulating agent (ESA) use. Both ESAs and prophylactic G-CSF were allowed from Cycle 2 onwards as clinically indicated. Therapeutic G-CSF, RBC, and platelet transfusions were allowed at any time during the studies as clinically indicated.
Study 1 was a randomized (1:1), double-blind, placebo-controlled study of COSELA or placebo administered prior to treatment with etoposide, carboplatin, and atezolizumab (E/P/A) for patients with newly diagnosed ES-SCLC not previously treated with chemotherapy. Study 1 was originally designed to evaluate Overall Survival (OS) as the primary efficacy endpoint comparing COSELA + E/P/A to placebo + E/P/A. However, based on the myelopreservation efficacy insignificant results of OS from Study 2, the protocol for Study 1 was amended prior to unblinding to update 2 co-primary and key secondary endpoints for assessing the drug’s effect in myelosuppression while retaining OS as a secondary endpoint. There were 2 co-primary myelosuppression efficacy endpoints: Duration of severe (Grade 4) neutropenia in Cycle 1 and occurrence of severe (Grade 4) neutropenia.
Study 2 was a randomized (1:1), double-blind, placebo-controlled evaluation of COSELA or placebo administered prior to treatment with etoposide and carboplatin (E/P) for patients with newly diagnosed ES-SCLC not previously treated with chemotherapy. Study 2 was designed to test COSELA’s mechanism of action in a clinical setting; it was a randomized (1:1), double-blind, placebo-controlled evaluation of COSELA or placebo administered prior to treatment with etoposide and carboplatin (E/P) for patients with newly diagnosed ES-SCLC not previously treated with chemotherapy. Based on the SAP, Study 2 has the statistical significance level set to be 0.20 for the comparison of treatment group differences. The sample size for this study was determined by clinical rather than statistical considerations. Therefore, the study was considered as exploratory. No inference should be made from the analysis.
Study 3 included a randomized, double-blind, placebo-controlled evaluation of COSELA or placebo administered prior to topotecan in patients with ES-SCLC previously treated with chemotherapy. Study 3 included a randomized, double-blind, placebo-controlled evaluation of COSELA or placebo administered prior to topotecan in patients with ES-SCLC previously treated with chemotherapy. According to the statistical analysis plan of Study 3, the primary comparison between two groups was not based on appropriate statistical significance level for testing between-group differences. The study was considered as exploratory. No inference should be made from the analysis.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.