Common Questions Related to Type II Veterinary Master Files (VMFs)
Type II Veterinary Master Files (VMFs) include chemistry, manufacturing, and controls (CMC) information (e.g., manufacturing process, controls, impurity profile information, specifications) to support the production of bulk drug substances, drug substance intermediates, medicated articles, medicated feeds, or finished dosage forms. This Q&A only covers the most common Type II VMF’s for bulk drug substances and drug substance intermediates. CVM has received numerous questions concerning Type II VMFs, ranging from administrative procedures (for example, When will my VMF be reviewed?) to specific content related to veterinary drug substances (for example, Is this an acceptable starting material?). This page is intended to provide answers to many common questions submitted by the holders of Type II Veterinary Master Files or their designated representative.
Although Type II VMFs are most commonly used for submitting CMC information on bulk drug substances and drug substance intermediates, this information can also be provided in the (abbreviated) new animal drug application [(A)NADA] or (generic) investigational new animal drug [(J)INAD] file for a drug product. Many questions in this Q&A apply to drug substance CMC information provided in a VMF, (A)NADA, or a (J)INAD; however, some questions (for example on the eSubmitter process) only apply to drug substance information provided in Type II VMFs.
Q3. For postapproval drug substance manufacturing changes reported in a Type II master file, how are the changes categorized?
Q4. What steps do master file holders as well as authorized users (i.e., drug product sponsors with a valid Letter of Authorization (LOA) from the master file holder) need to take after minor CMC changes are made for a drug substance?
Q5. What steps do master file holders as well as authorized users need to take after moderate or major CMC changes are made for a drug substance?
Q6. What steps do master file holders as well as authorized users need to take if a master file holder references another master file that contains moderate or major changes?
Q7. After moderate or major changes are reported for a Type II master file, does CVM immediately review these changes in the master file?
Q8. Can a sponsor submit the same information to multiple affected drug products that all use the same drug substance with the reported moderate or major changes?
Q9. What actions may CVM take if moderate or major changes in a Type II master file are not reported by a referencing drug product sponsor?
Q10. What are other sources of information on postapproval drug substance changes reported to a Type II master file?
Q12. When will my Type II master file be reviewed?
Q13. When will I learn the status of my master file?
Q14. Does CVM re-review master file submissions?
Q15. What is the cGMP status of my drug substance manufacturer?
Q16. What guidance documents do I look at regarding what to submit for a new Type II VMF?
Q17. Which VMF Submission Type should I select for this submission (A, C, G, E, Z, or Y)?
Q18. For a VMF Quality (C) Submission Type, which Submission Classification Code should I select?
Q19. How do I get a VMF number for a new VMF through eSubmitter?
Q20. Do I need to submit my VMF in eCTD format the same way I do for a DMF?
Q21. How do I submit an amendment to a currently open “C” submission?
DRUG SUBSTANCE, LABELING
Q1. What should be on a drug substance container label?
The label affixed to the container closure system of the drug substance should include the name of substance, manufacturer's name, address of manufacturing site, expiration or retest date as appropriate (see ICH Q1A(R2) Stability Testing of New Drug Substances and Products and Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients), batch number, storage conditions with a numeric temperature range supported by long-term stability data, and other storage statements (e.g., excursions, protect from light) supported by stability data (e.g., accelerated stability data, forced degradation studies) as needed.
DRUG SUBSTANCE, MANUFACTURING PROCESS
Q2. Will CVM accept this starting material (SM)? What criteria does CVM use to evaluate an SM?
We recommend you identify the proposed regulatory starting materials in the drug substance manufacturing process and include justification in the master file to support the selection of those regulatory starting materials. CVM utilizes the following criteria to evaluate the regulatory starting material: the complexity of the structure, carryover of impurities, and propinquity. An SM should be a well-characterized and purified, contributes to the final drug substance’s structure, and is often commercially available material that is also used outside of the pharmaceutical industry. For more details, refer to GFI #169 Drug Substance Chemistry, Manufacturing, and Controls Information as well as the principles found in ICH Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients and ICH Q11 Development and Manufacture of Drug Substances. Please note that without an assessment of the full chemistry, manufacturing, and controls (CMC) information for the drug substance (e.g., manufacturing process, controls, impurity profile information, specifications), it is not possible to determine whether the proposed starting material is appropriate. Therefore, the determination of the acceptability of starting materials in informal communications and/or meetings with CVM is not feasible.
DRUG SUBSTANCE, POSTAPPROVAL CHANGES
Q3. For postapproval drug substance manufacturing changes reported in a Type II master file, how are the changes categorized?
Postapproval drug substance manufacturing changes may or may not affect the quality of the drug product. The changes are categorized as minor (21 CFR 514.8(b)(4)), moderate (21 CFR 514.8(b)(3)), or major (21 CFR 514.8(b)(2)). In general, the category of a change is determined by the risk the change poses to the identity, strength, quality, purity, and/or potency of the drug product as these factors may relate to its safety or effectiveness. The changes reported in Type II master files for a drug substance are categorized by the effects that these changes may have on the final drug product.
Assessment of the possible effects that result from a drug substance manufacturing change is important. Evaluation of the scope of the change, the process context in which the changes are made, and the determination of pre- and post-change equivalence are critical aspects of the assessment. The risk and therefore the reporting category increase for manufacturing changes that occur later in the process, especially after the final intermediate or within the final processing step; such changes carry a higher risk of altering the quality of the drug product.
For example, a change to new manufacturing equipment with the same operating principle, design, and materials of construction is generally considered a minor change. However, a change to new equipment with different operating principles, design, or materials of construction may be considered a moderate change. Furthermore, if the equipment change occurs after the formation of the final intermediate, it may be considered a major change.
Another example of moderate or major changes to drug substance manufacturing include changes in the synthetic route. If the change in the synthetic route occurs early in the manufacturing process, it may be considered a moderate change; however, if the change in the synthetic route occurs at the final intermediate, it may be considered a major change.
Determination of pre- and post-change equivalence may include evaluation of the physical properties and impurity profiles at relevant steps in the manufacturing process.
For additional information on the reporting category for manufacturing changes in Type II master files, please refer to CVM GFI #83 Chemistry, Manufacturing, and Controls Changes to an Approved NADA or ANADA and the other references in Question 10.
Q4. What steps do master file holders as well as authorized users (i.e., drug product sponsors with a valid Letter of Authorization (LOA) from the master file holder) need to take after minor CMC changes are made for a drug substance?
Actions the master file holder should take
The holder of the master file should submit an amendment to the file with supporting information for the CMC change(s). In addition, the holder should notify all authorized users of the CMC change(s), because sponsors of drug products have a regulatory requirement under 21 CFR 514.8(b)(4) to assess the effects of the drug substance changes on their drug products.
Actions the master file’s authorized users should take
For minor drug substance CMC changes, authorized users should provide the appropriate reference to the master file as well as report their assessment, if any, of the effects of the changes on their drug product in their next Minor Changes and Stability Report (MCSR) for their drug product application(s).
Q5. What steps do master file holders as well as authorized users need to take after moderate or major CMC changes are made for a drug substance?
Actions the master file holder should take
The holder of the master file should submit an amendment to the file with supporting information for the CMC change(s). In addition, the holder should notify all authorized users of the CMC change(s), because sponsors of drug products have a regulatory requirement under 21 CFR 514.8(b)(2) to assess the effects of the drug substance changes on their drug products. If the authorized users are not notified of moderate or major CMC changes within the Type II master file, then regulatory approvals and subsequent marketing of the drug product(s) could be delayed. Timely and effective communication between master file holders and authorized users is highly encouraged.
Actions the master file’s authorized users should take
After being informed of a moderate or major CMC change to a drug substance, the authorized user should assess the effects of the reported change(s) on the identity, strength, quality, purity, and potency of their drug product as these factors may relate to safety or effectiveness. The authorized user should then submit the appropriate supplement to CVM for the relevant application(s) as required by 21 CFR 514.8(b)(2).
The supplement should include an appropriate reference to the master file as well as their assessment of the effects of the CMC change(s) on their drug product. The extent of the assessment, as well as the type of supplement [Prior Approval Supplement (PAS) for major changes; Supplement—Changes Being Effected in 30 Days (CBE-30) or Supplement—Changes Being Effected (CBE-0) for moderate changes] is determined by the nature of the change and the risk of potential adverse effects on the identity, strength, quality, purity, or potency of their drug product.
The characteristics of the drug product will influence the assessments that are required.
For example, changes in the particle size or flowability of a drug substance powder will likely necessitate evaluation for drug products that are not solubilized during the manufacturing process (e.g., tablets, suspensions, dry Type A medicated articles).
Drug substance changes that require the submission of a Prior Approval Supplement (PAS)
For drug substance changes that require the submission of a PAS, a drug product cannot be manufactured (utilizing the drug substance with the manufacturing changes) for commercial use or distributed before the supplement is approved by CVM; doing so may result in the product being considered adulterated according to Section 501(a)(5) of the Federal Food, Drug, and Cosmetic Act.
Drug substance changes that require the submission of a CBE-30
For drug substance changes that require the submission of a CBE-30, a drug product may be manufactured (utilizing the drug substance with the manufacturing changes) at the sponsor’s own risk and distributed for commercial use if not notified otherwise by CVM after 30 days. However, if CVM determines that the master file is deficient and the CBE-30 supplement is incomplete, then continued marketing of the drug product may result in the product being considered adulterated according to Section 501(a)(5) of the Federal Food, Drug, and Cosmetic Act.
Drug substance changes that require the submission of a CBE-0
For drug substance changes that require the submission of a CBE-0, a drug product may be manufactured (utilizing the drug substance with the manufacturing changes) at the sponsor’s own risk and distributed for commercial use immediately. However, if CVM determines that the master file is deficient and the CBE-0 supplement is incomplete, then continued marketing of the drug product may result in the product being considered adulterated according to Section 501(a)(5) of the Federal Food, Drug, and Cosmetic Act.
Q6. What steps do master file holders as well as authorized users need to take if a master file holder references another master file that contains moderate or major changes?
Actions the master file holder should take
If a master file holder references another master file that contains moderate or major changes, then the holder of the primary master file should assess the effects, if any, on their manufacturing process as well as on the final drug substance and report the change(s) as appropriate to their authorized users.
Actions the master file’s authorized users should take
The authorized users should follow the actions outlined above as per the information provided by their referenced master file holder.
Q7. After moderate or major changes are reported for a Type II master file, does CVM immediately review these changes in the master file?
Master files that contain moderate or major changes are not reviewed until a drug product supplemental application that references the master file is received by CVM. Master file holders should be promptly communicating moderate or major changes to their authorized users, who would need to submit the appropriate supplement that addresses the effects, if any, of the drug substance manufacturing changes on their drug product.
Note that in cases where multiple drug product applications reference the Type II master file, each application is required to submit a supplement that addresses the moderate or major drug substance manufacturing change(s) with respect to that drug product. Even if another application has initiated the review of the major or moderate changes in a master file, it is still necessary to evaluate the effects of these drug substance changes on each referencing drug product (see Question 14). In certain circumstances, a single sponsor with multiple referencing applications may be able to file the identical information to support the drug substance manufacturing change(s) in more than one application (see Question 8).
Q8. Can a sponsor submit the same information to multiple affected drug products that all use the same drug substance with the reported moderate or major changes?
Yes, this may be possible. If a sponsor owns multiple drug product applications that reference the same master file, and product-specific data does not need to be generated to support the change(s), then identical supplements could be submitted for all referencing drug products. In this case, a linked submission covering all the products should be created using CVM’s electronic submission system (eSubmitter).
If product-specific data needs to be generated for different applications, then the supplements should not be linked but instead should be filed separately.
For example, if an authorized user has two applications, one a tablet, the other a true solution, that reference the same master file, and the manufacturing change to the drug substance altered the particle size, then the authorized user should provide separate supplements to the applications because the changes to the particle size may affect manufacturability of the dosage forms differently.
Q9. What actions may CVM take if moderate or major changes in a Type II master file are not reported by a referencing drug product sponsor?
If a Type II master file holder submits moderate or major changes to CVM and the authorized user(s) have not submitted a corresponding supplement for the affected drug products, letters will be issued to the authorized users to notify them of their obligation to submit a supplement to their drug product application(s). A letter will also be issued to the master file holder informing them to notify their authorized users of the moderate or major changes reported in the master file.
Review of the master file changes will only commence after a referencing authorized user submits the appropriate supplement. If an authorized user does not submit a supplement for the affected products, drug product manufactured with batches of the drug substance made with moderate or major changes may result in the product being considered adulterated according to Section 501(a)(5) of the Federal Food, Drug, and Cosmetic Act.
Q10. What are other sources of information on postapproval drug substance changes reported to a Type II master file?
Additional information on this subject can be found in the following references:
- 21 CFR 514.8
- CVM GFI #57 Preparation and Submission of Veterinary Master Files
- CVM GFI #83 Chemistry, Manufacturing, and Controls Changes to an Approved NADA or ANADA
- CVM GFI #126 BACPAC I-Intermediates in Drug Substance Synthesis Bulk Actives Postapproval Changes: Chemistry, Manufacturing, and Controls Documentation
DRUG SUBSTANCE, STABILITY
Q11. What is an appropriate stability protocol and post-approval stability commitment for a drug substance?
The principles in CVM GFI #5 Drug Stability Guidelines can be applied to drug substances. In addition, section 2.3.S.7 of CVM GFI #234 Question-Based Review for the Chemistry, Manufacturing, and Controls Technical Section of Animal Drug Applications contains specific guidance for the information that should be included for a drug substance stability protocol and post-approval stability commitment. Drug substance stability is also addressed in CVM GFI #73 (VICH GL3(R)) Stability Testing of New Veterinary Drug Substances and CVM GFI #259 (VICH GL58) Stability Testing of New Veterinary Drug Substances and Medicinal Products in Climatic Zones III and IV.
As stated in the guidance documents above, a drug substance stability protocol should address the following points:
- The stability specification (chemical, microbiological, and physical tests, methods, and the corresponding acceptance criteria) that will be used
- Numeric temperature and humidity storage conditions and the testing intervals
- A description of the stability packaging (if it differs from the commercial packaging)
- Retest or expiry period
The guidance documents also describe the following points that should be addressed in a drug substance post-approval stability commitment:
- A commitment to report updated stability data annually to the master file
- A commitment to place new batch(es) on stability each year or provide a statement explicitly stating that no batches were manufactured during that year
- A commitment to test the first batch(es) after a major manufacturing change (i.e., a change to the process, facility, or container closure system) according to your original stability protocol (i.e., testing intervals every 3-months for the 1st year, every 6-months for the 2nd year, and annually thereafter)
- A commitment to investigate any batches found to be out-of-specification (OOS) and take appropriate action on the impacted batch(es)
- A commitment to notify all authorized users of any OOS results
MASTER FILE, GENERAL QUESTIONS
Q12. When will my Type II master file be reviewed?
Master files are reviewed when they are appropriately referenced by an (Abbreviated) New Animal Drug Application ((A)NADA), (Generic) Investigational New Animal Drug File ((J)INAD), or another Master File (MF). Only the VMF submissions submitted before or at the same time as a referencing submission will be reviewed. Thus, the due dates for the review of these VMFs will correspond to that of the referencing submission. VMF holders may reach out to their authorized users to inquire about the due date of their referencing submission(s) to determine when the VMF would be reviewed. Please note that when manufacturing changes are made to the master file, the VMF holder should contact their authorized users to ensure they are aware of the change and have referenced the VMF with an appropriate postapproval submission (annual report or supplement) to their (A)NADA.
In cases where a VMF is regularly referenced appropriately by a drug product’s Minor Changes and Stability Report (MCSR) in the eSubmitter template, and only minor changes have been reported in the VMF, then the VMF submission(s) may undergo routine review by CVM, generally within 180 days of submission. Routine review is intended to assess minor updates to a VMF to expedite the review process. However, if a VMF submission contains moderate or major manufacturing changes, all authorized users should file a supplement to assess the effects of these changes on their drug product as well as to initiate the review of the VMF. For further information, please refer to Question 5 as well as to the CVM Program Policy and Procedures Manual 1243.2400 Veterinary Master Files with Manufacturing Information.
Q13. When will I learn the status of my master file?
VMFs are not approved. Instead, master files are reviewed for adequacy to support (A)NADAs, (J)INADs, or another master file. If the review finds the master file adequate, but additional information is needed, an information request letter is issued; if the review finds the master file inadequate (or deficient), a deficiency letter is issued. For master files found to be adequate to support a referencing submission, no letter is issued to the firm. Master files that are not referenced are not reviewed and no letter is issued to the firm regarding status.
Q14. Does CVM re-review master file submissions?
In general, to increase efficiency and maintain consistency, master file submissions, both Veterinary Master Files (VMFs) and Drug Master Files (DMFs), are not re-reviewed by CVM. However, in certain situations, CVM may need to re-review a master file submission.
If the intended dosage form of the drug product is different, some information submitted to a master file may need to be reassessed. For example, the polymorphic form of the drug substance may not be a critical quality attribute for true solution. If the drug substance is later proposed for use in a tablet, then a re-evaluation of the drug substance specifications and physicochemical characterization may be necessary to ensure that the physical properties of the drug substance do not negatively impact the drug product.
If impurities in the drug substance affect humans and other animal species differently, CVM may review the impurities characterization and control information from a master file again to determine whether the target animal(s) for the corresponding drug product would be adversely affected. Additionally, if the same drug substance were to be used to treat multiple species or different patient populations, then the master file may need to be reevaluated, taking into account the maximum daily dosing of each end user.
The examples above may not include all situations where information that was reviewed in a master file would be reviewed again.
Information concerning the review of CMC information that is provided to FDA for both human and animal drugs can be found at Chemistry, Manufacturing, and Controls (CMC) Information Submitted to Both CDER and CVM.
Q15. What is the cGMP status of my drug substance manufacturer?
FDA discloses the inspectional outcome of regulated facilities on the FDA Data Dashboard. Facilities that have registered as a drug manufacturing establishment (but have not necessarily been inspected) may be found at Drug Establishments Current Registration Site. Additional information about the registration process can be found at Registration and Listing. For further information about cGMP or registration status of a drug substance manufacturer’s facility beyond what is available at the sites above, please contact the firm directly.
Q16. What guidance documents do I look at regarding what to submit for a new Type II VMF?
Please refer to GFI #57 Preparation and Submission of Veterinary Master Files, for general information about submitting VMF submissions. Note that VMF submissions should be submitted electronically via eSubmitter; thus, the GFI #57 sections pertaining to paper submissions are not current. Guidance on CMC aspects of VMF submissions can be found on Chemistry Manufacturing and Controls (CMC) Guidances for Industry (GFIs) and Questions and Answers (Q&As).
MASTER FILE, ESUBMITTER PROCESS
Q17. Which VMF Submission Type should I select for this submission (A, C, G, E, Z, or Y)?
An “A” submission is used to establish and obtain a number for a new VMF, and it should not include any CMC information. See Q19 for additional information for establishing a new VMF. “A” submissions are most often sent to the Division of Manufacturing Technologies (HFV-140) for review.
A “C” submission contains the original CMC information; a total update; minor (annual reports), moderate, or major CMC changes; and responses to CVM CMC letters. “C” submissions are sent to the Division of Manufacturing Technologies (HFV-140) for review. See Question 18 for explanation of further Classification Codes required for these types of submissions.
A “G” submission is used for requests to terminate a VMF and for other administrative information and requests (e.g., letters of authorization, firm name changes, agent update transfer of ownership). These submissions should not include any CMC information. “G” submissions are most often sent to the Division of Business Information Science and Management (HFV-180) for firm name change requests or the Division of Manufacturing Technologies (HFV-140) for other actions.
An “E” submission is a protocol (with no data) to a VMF. This may be used to request concurrence from CVM on method procedures or stability protocols before implementation. “E” submissions are most often sent to the Division of Manufacturing Technologies (HFV-140) for review.
A “Z” submission is a submission to request either a pre-submission conference or other meeting with CVM under a VMF. “Z” submissions are most often sent to the Division of Manufacturing Technologies (HFV-140) for scheduling.
A “Y” submission is a request for revisions to the Memorandum of Conference from a conference or meeting with CVM. “Y” submissions are most often sent to the Division of Manufacturing Technologies (HFV-140) for review and corrections.
Q18. For a VMF Quality (C) Submission Type, which Submission Classification Code should I select?
An “AC” classification code is for a submission, such as an annual report, that provides stability data and minor changes to the chemistry, manufacturing, and controls (CMC) information.
An “MC” classification code is for a submission that contains major or moderate CMC changes that should be accompanied by a referencing drug product supplement.
An “MR” classification code is for a firm’s response to a CMC letter from CVM.
An “OT” classification code is for an original submission or a total update to a VMF. Most often a total update is used when revising large portions of the file, for example when converting a VMF to CTD (Common Technical Document) format or when substantial portions of each subsection are needed. Note that for OT submissions, entries into each of the eSubmitter subsections is required to send a submission.
Q19. How do I get a VMF number for a new VMF through eSubmitter?
CVM does not pre-assign VMF numbers. An “A” submission in eSubmitter is used to obtain a number for a new VMF. As part of this initial submission, we recommend including a letter requesting the establishment of a VMF. An e-mail acknowledging successful transmission of the submission should arrive within a few minutes and will contain the new VMF number. Note that when the VMF is opened, the subsequent “C” submission to provide the full chemistry, manufacturing, and controls (CMC) information should be submitted as an Original, Total Update (“OT” Submission Classification Code) through the eSubmitter tool, which will allow access to the question-based review (QbR) sections that will need to be populated.
Please note that CVM will not initiate a CMC review of a VMF until full CMC information is submitted to the VMF in a “C” submission subsequent to the initial “A” submission.
Q20. Do I need to submit my VMF in eCTD format the same way I do for a DMF?
The CVM VMF submission process is different than the CDER Drug Master File (DMF) submission process. We recommend using Common Technical Document (CTD) format for VMFs. The question-based review (QbR) sections in eSubmitter are structured to build CTD-like submissions of text or PDF attachments.
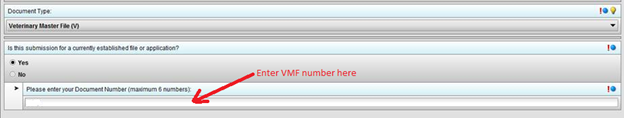
Q21. How do I submit an amendment to a currently open “C” submission?
To submit an amendment to an open submission, you can enter these selections in the 1.0 Document Information screen:
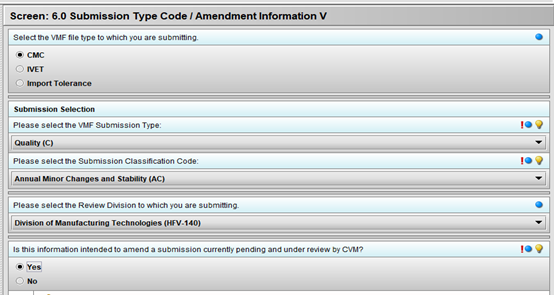
Advance using the green arrow button to the 2.0 Firm Information screen and fill in these fields if you have not already done so. Next, advance to Screen 6.0 Submission Type Code / Amendment Information V and make these selections:
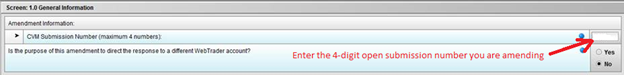
Selecting ‘Yes’ for “Is this information intended to amend a submission currently pending and under review by CVM?” allows access to Screen 1.0 General Information when you advance. Select these responses on this screen:
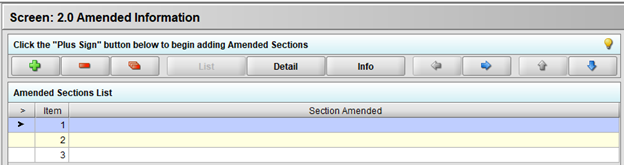
After advancing this time to the Screen 2.0 Amended Information, on the next screen click the green plus sign button and you should then be able to add files in the Amendment tab:
Here, you may attach your documents as an Adobe Acrobat (.PDF) files using the green plus sign button.