Bioresearch Monitoring Program FY2015 Program Alignment Specialization Action Plan

Introduction
The FY2015 BIMO Specialization Action Plan is being collaboratively developed by a working group with members representing the Center for Biologics Evaluation and Research (CBER), the Center for Drug Evaluation and Research (CDER), the Center for Devices and Radiological Health (CDRH), the Center for Food Safety and Applied Nutrition (CFSAN), the Center for Tobacco Products (CTP), the Center for Veterinary Medicine (CVM), the Office of Good Clinical Practice (OGCP), the Office of Global Regulatory Operations and Policy (OGROP) and the Office of Regulatory Affairs (ORA). The working group is tasked with evaluating and recommending how specialization will be defined and implemented for the FDA Bioresearch Monitoring (BIMO) program.
The working group believes, first and foremost, a specialized BIMO program with dedicated BIMO investigators will result in higher quality BIMO inspections. Increased specialization and dedication of investigators in the BIMO program will result in investigators gaining more in-depth knowledge of BIMO regulations, policies and industry practices related to BIMO, and therefore, will allow them to refine the unique skills needed to conduct these inspections. Specialization and dedication will also provide for an enhanced professional development program for ORA investigators, including a robust curriculum of specialized training and practical experience for ORA investigators interested in specializing in BIMO or ascending to management positions within the program.
Background on the BIMO program
The overarching goals of the agency’s BIMO program are to protect the rights, safety, and welfare of subjects involved in FDA-regulated clinical trials; to determine the accuracy and reliability of clinical trial data submitted to FDA in support of research or marketing applications; and to assess compliance with FDA’s regulations governing the conduct of clinical trials, including regulations for informed consent and ethical review. The BIMO program includes inspections related to Sponsors, Contract Research Organizations, Monitors, Clinical Investigators, Institutional Review Boards, in-vivo Bioequivalence, Good Laboratory Practice (nonclinical laboratories), Good Laboratory Practice program EPA Data Audit Inspections, and Radioactive Drug Research Committees.
FDA’s BIMO program was developed by a cross center collaborative group representing all commodity areas in the 1970’s following the promulgation of the Investigational New Drug regulations in 1963. The program continues to be unique in FDA as it includes multiple product centers, OGCP, and ORA working collaboratively to support the regulatory review process, protect the rights, safety and welfare of human subjects participating in clinical trials, and to verify the accuracy and reliability of clinical trial data submitted in support of product applications.
Currently, each product center is responsible for identifying sites for inspection, supplying Subject Matter Experts to assist in a small subset of complex inspections, determining the final classification of each inspection, and taking compliance action as needed. ORA staff assigns investigators to conduct inspections, prepares the inspection reports, and monitors assignments in each district. Several districts have one or two BIMO Specialists (investigators who have expert knowledge in and work almost exclusively in the BIMO program), but most investigators conducting BIMO inspections are not dedicated to this program area. Most investigators conduct BIMO inspections in addition to their primary program area.
Although each center manages its own BIMO program, OGCP, in the Office of the Commissioner, facilitates the harmonization of policies and practices across all centers through collaborative efforts in policy development and promoting the sharing of best practices. The BIMO program is a cross-center regulatory program with well-defined leads, a well-structured policy development process, and a well-established governance system. The recent survey of relevant FDA staff described in the document entitled “Inventory of FDA Compliance Activities: Hot Spots and Best Practices” repeatedly identified the BIMO compliance program as a best practice.
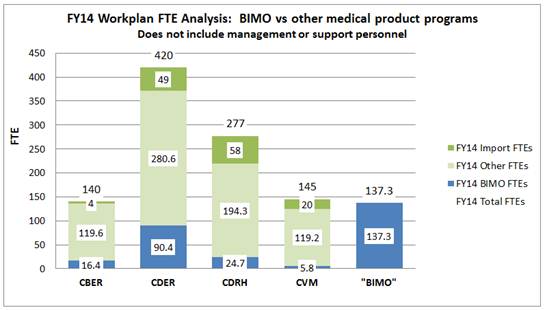
Figure 1 compares the BIMO program’s ORA FTEs to other medical product programs using the FY2014 ORA Workplan. (Note: CFSAN restructured its BIMO program in FY2014 and plans to allocate additional FTEs for CFSAN BIMO). As demonstrated by Figure 1, the BIMO program includes 137 FTE spread across CBER, CDER, CDRH and CVM. This number of FTE is comparable to the FTE count dedicated to the entire CBER (140) and CVM (145) programs. Note that the BIMO program is actually larger than the CBER program when BIMO resources are deducted.
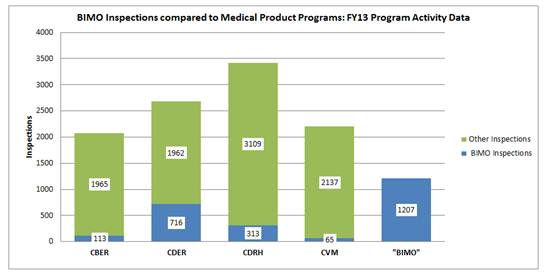
Figure 2 compares the total number of BIMO and non-BIMO (labeled as “other”) inspections done for CBER, CDER, CDRH and CVM in FY2013. (Note: CFSAN restructured its BIMO program and has performed 3 BIMO inspections in FY2014). As demonstrated by Figure 2, the total number of BIMO inspections in FY2013 was 1207 inspections. Although smaller in quantity than the number of “other” inspections conducted for these centers, the working group believes the number of BIMO inspections conducted annually is more than enough to justify increased levels of specialization and dedication of ORA investigators for the BIMO program.
Specifics of the BIMO Action Plan
The BIMO Action Plan working group quickly reached general consensus that the BIMO program is functioning reasonably well but would benefit from creating an enhanced program for BIMO specialization and the dedication of a corps of ORA investigators to conduct BIMO inspections. Additional preliminary recommendations have been considered however; exactly how specialization and dedication is defined and implemented will require additional analysis and discussion by the working group. The working group plans to conduct this analysis in FY2015. For instance, the working group strongly agreed that the training and experience needed to conduct BIMO inspections is applicable to all BIMO inspections required by all centers, with the exception of CTP. Similarly, it was felt by the working group that it is more important for the ORA investigators to be familiar with the GCP and GLP requirements for conducting and overseeing FDA-regulated research and human subject protection than to have product-specific expertise for the conduct of BIMO inspections. Other aspects of specialization will require additional analysis (e.g., whether specialization should be specific to one inspection type, multiple inspection types, or all BIMO inspection types; amount of time specialists dedicate to BIMO inspections). As such, the working group recommends that a cross-commodity ORA BIMO program be structured similarly to the single commodity based programs per the Commissioner’s PAG Memo: “Specialized units in ORA operating in program-based staffs will be directed and managed by commodity-specific offices and led by a senior executive.”
The working group agrees that a critical component for the successful implementation and management of the ORA BIMO program is the appointment of a single leader, with the appropriate authority, for the ORA BIMO program. Ideally, the creation of this new leadership position would be on a similar schedule as the new commodity-specific program leadership positions being planned. Additionally, the working group endorses the creation of a dedicated inspection workforce (i.e., ORA investigators whose workload is dedicated to conducting BIMO inspections for multiple FDA centers with agreed upon leeway for emergencies). It should be noted, however, that all Tobacco BIMO inspections will be conducted by the ORA Tobacco Cadre, as tobacco products are uniquely regulated by FDA and have a different standard of review than the other FDA-regulated products.
The working group’s recommendations for the specialized ORA BIMO program are based on the following:
- The expertise required to conduct BIMO inspections includes clinical and nonclinical (i.e., pre-clinical) knowledge and this needed expertise differs markedly from the other inspection programs (manufacturing, labeling, imports, etc.). Additionally, the entities inspected are generally different for BIMO compared to the other types of inspection programs.
- Cross-commodity BIMO inspections are more similar to each other than to related commodity inspections. For example, an inspection of a sponsor of a clinical trial involving a drug product is more similar to an inspection of a sponsor of a clinical trial involving a device than to a drug manufacturer inspection. In fact, the compliance program guidance manuals (CPGMs) for most BIMO inspection programs (e.g., IRB, CI, Sponsor/CRO, etc.) were developed through collaborative efforts across all centers and are used by ORA investigators for the conduct of the BIMO inspections for all centers irrespective of commodity type.
- The size of an ORA BIMO program is comparable to other inspectional programs. The FY2013 Program Activity Data reports 1207 BIMO inspections. The FY2014 ORA Workplan allocated 137 FTEs to BIMO. (See Figures 1 and 2)
The working group recognizes that some aspects of the cross-commodity ORA BIMO program will necessarily differ from the single commodity programs. For instance, extensive cross-commodity collaboration will be required for training, compliance, and work planning. The foundation for such cross-commodity collaboration already exists through the efforts of OGCP and ORA and their work on facilitating cross-center collaboration on policy development, training, and the sharing of best practices. All centers and the ORA BIMO program staff regularly contribute to these efforts.
In FY2015, the working group will conduct an analysis of the existing BIMO program, and make more specific recommendations to facilitate increased operational and program alignment, as FDA transitions to more vertically-integrated regulatory programs. The working group will evaluate and identify the skills, training, and competencies needed to support increased program specialization. The group will also evaluate the processes associated with resource planning, policy development, compliance activities, communication among the centers and with ORA, training, and outreach to identify opportunities to improve operational efficiencies and alignment within the program.
Initially, the Program Alignment BIMO Working Group will focus on the following three areas for analysis and discussion: (1) options related to BIMO specialization, (2) competencies and training required for BIMO specialization and (3) how to best manage the processes connected to the BIMO program. Below, we identify targeted actions items in each of these three areas. All actions will be completed in FY2015 and more specific target dates for milestones will be established upon implementation of the plan.
A. BIMO Specialization
- Develop a paper describing different BIMO specialization options that support increased operational and program alignment as FDA transitions to more vertically-integrated regulatory programs. This options paper will include an analysis of pros and cons related to how BIMO specialization is defined and implemented and will consider issues such as competencies, training, resource needs, resource usage, and potential areas of sub-specialization (sponsor, IRB, BE, etc.). The target date for completion is April 1, 2015.
- Define the program’s goals and elements necessary to maintain a successful stand-alone BIMO program. Such elements may include the establishment and filling of key positions needed for liaison activities across FDA organizations and with external partners; development and execution of surveillance workplans for Non-Clinical Laboratories and Institutional Review Boards; and improved communications between center programs and ORA field investigators. Additional elements will be considered as needed.
- Identify and describe the required areas of specialization and the associated general competencies for investigators in these areas of specialization. This analysis shall include the review of applicable regulations and compliance programs (Clinical Investigator, Sponsor/Monitor/CRO, Institutional Review Board, Radioactive Drug Research Committee, Clinical Bioequivalence, Clinical Endpoint Bioequivalence, Bioanalytical Bioequivalence, Good Laboratory Practice, etc.).
- Conduct an analysis to support the identification of program and specialization area resource needs versus current resource capacity. This analysis will include (among other things): inspection and assignment planning; volume of inspections; number of individual investigators conducting BIMO inspections; inspection time requirements; geographic distribution of BIMO sites; and FTE allocation. The data will be broken down by each center. Additionally, an analysis of the current level of specialization of ORA investigators in terms of knowledge and time spent on BIMO versus other inspection types will be conducted.
- Evaluate other existing compliance programs for possible clinical/nonclinical components (Postmarketing Adverse Drug Experience, Risk Evaluation and Mitigation Strategies, Drug Postmarketing Requirements, Device Postmarket Surveillance Studies, CVM Genetically Modified Organisms) for possible inclusion in the BIMO program.
B. BIMO Competencies and Training
- Develop strategies for recruitment, hiring, retention, and succession planning across the program.
- Develop competencies to be used as benchmarks for BIMO investigator hiring.
- Analyze currently available training and certification programs to define the new specialized training curricula (including certification) based on areas of specialization that will include program and subject matter experts. This data will also be used to identify possible training gaps and/or opportunities to leverage across FDA.
- Develop a recommendation for a consolidated training program for BIMO investigators and center BIMO staff. The training program will include center participation in training development and administration, center participation in inspections, and possible opportunity for investigator training visits to the centers.
C. BIMO Program Processes
- Review the current work planning model and evaluate the relationships among the program’s resources, activities and current processes. Identify the areas within the work plan that require revision to address program specialization and improve the alignment of the program’s resources and activities. The new resource planning model will account for surveillance, for cause, and application driven work and will improve inspection allocation to each center to ensure its BIMO activities are adequately resourced. The model will also consider the global nature of FDA’s work (e.g., foreign inspections, movement toward mutual reliance on other regulatory agencies’ inspectional findings), and other future needs as they are identified.
- Identify and analyze additional program work-flow procedures and processes, to include regulation and policy development, as appropriate, enforcement and compliance activities, communications (e.g., between center reviewers and investigators), outreach and information sharing, and electronic recordkeeping and dissemination of assignment related information from applications, with an eye to create more proactive, efficient and inclusive processes with clearly defined roles and responsibilities.
- Utilize the work performed in #C2 to identify and address any current gaps in program procedures and processes.