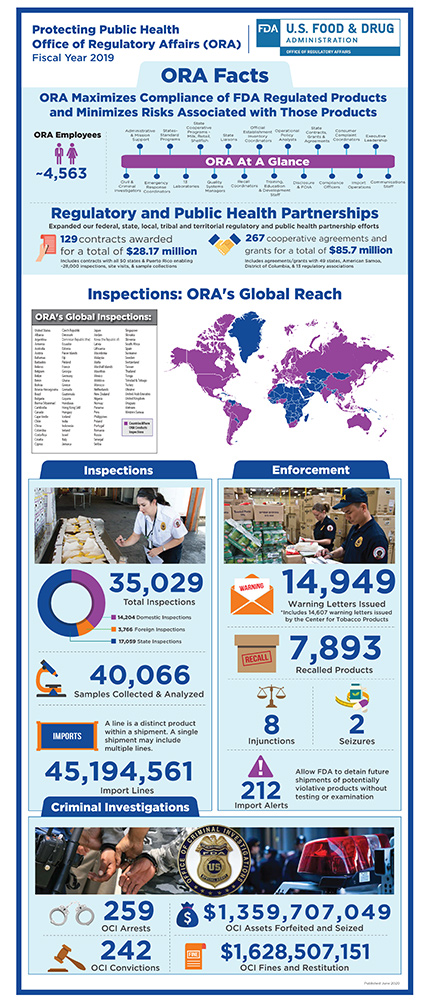
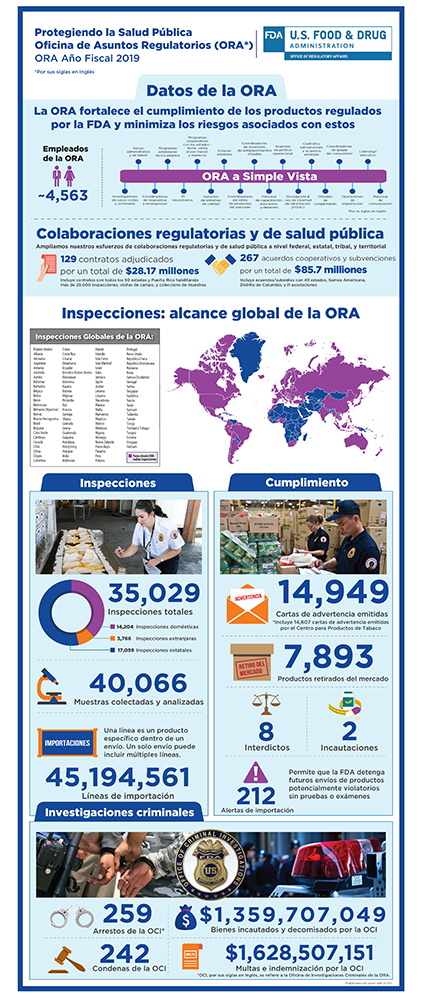
FDA Efforts to Ensure Product Safety
The Food and Drug Administration uses a robust, risk-based oversight approach to ensure the continued safety of the nation’s food and medical product supply. This multi-pronged approach includes:
- inspections,
- investigations, including criminal investigations
- sample collections and testing,
- reviews of imported packages offered for entry into the U.S.
- remote regulatory assessments
- information from capable foreign regulatory partners where there are mutual recognition agreements for drugs,
- information from trusted foreign regulatory partners under the single audit program for medical devices, and
- information from trusted foreign regulatory partners where we have systems recognition for foods.
FDA Oversight During the COVID-19 Pandemic
The COVID-19 pandemic created an unprecedented public health emergency that impacted FDA’s inspectional work. The agency’s expanded use of a variety of surveillance tools and development of new oversight approaches significantly contributed to our ability to protect and promote public health, while also protecting the safety of FDA staff, employees at the firms FDA inspects, and the public.
FDA issued a report, “Resiliency Roadmap for FDA Inspectional Oversight,” in May 2021, which detailed the effect of the COVID-19 pandemic on inspectional activities and outlined our plan for returning to a more consistent state of conducting oversight of regulated industry.
FDA issued an updated report in November 2021, which provides information on the current state of FDA oversight, and focuses on how we have exceeded the goals set in May 2021 for completing domestic surveillance inspections in FY2021.
FY21 Highlights
- Exceeded its goals for completing domestic surveillance inspections as outlined in the Resiliency Roadmap’s “base-case scenario.”
- Completed inspections and/or assessments in support of all seven of the identified mission critical applications.
- Made decisions on nearly half of the 68 applications reported as delayed due to a pending inspection or assessment in the Resiliency Roadmap.
- Exceeded its goal related to following up on previous inspections classified as official action indicated (OAI).
- Made more than 1,300 requests to human and animal drug and biological product manufacturers to submit records for review, which supported timely regulatory decisions.
- Conducted more than 130 remote regulatory assessments under the bioresearch monitoring program that were directly used in application decisions.
For More Information
- Integrated Food Safety System (IFSS)
- Domestic mutual reliance
- An update to the Resiliency Roadmap for FDA Inspectional Oversight (November 2021)
- The Resiliency Roadmap for FDA Inspectional Oversight (May 2021)
- Press release: The Resiliency Roadmap for FDA Inspectional Oversight (May 5, 2021)
- FDA Voices articles on the importation of food and medical products, how we protected patients and consumers from fraud and our inspectional tools during the pandemic
Previous Oversight Highlights