Real-Time Oncology Review
Purpose of the RTOR
The Oncology Center of Excellence Real-Time Oncology Review (RTOR) aims to explore a more efficient review process to ensure that safe and effective treatments are available to patients as early as possible, while maintaining and improving review quality and balancing the review team’s workload through data and analysis standardization, and early iterative engagement with the applicant.
Scope
New Drug Application (NDA) and Biologics License Application (BLA) submissions will be selected from each clinical division (DO1, DO2, DO3 and DHM1 and DHM2) to evaluate the feasibility and to optimize the process for RTOR. Acceptance into the RTOR does not guarantee or influence approvability of the application, which is subject to the usual benefit-risk evaluation by FDA reviewers. Participation by the applicant in this program is voluntary.
It is not the intent of the RTOR to enable an early action prior to the specified user fee goal date.
Submissions to be considered for RTOR should meet the following criteria:
- Drugs likely to demonstrate substantial improvements over available therapy, which may include drugs previously granted breakthrough therapy designation for the same or other indications.
- Drugs meeting other criteria for other Expedited Programs (e.g. fast track, priority review) may also be considered as described in the guidance.
- Straightforward study designs, as determined by the review Division and the OCE.
- Endpoints that can be easily interpreted (e.g. overall survival, progression free survival, etc.).
Submissions with greater complexity will be considered for RTOR on a case by case basis. Additionally, FDA generally prefers the Assessment Aid (AAid) be used with RTOR.
Standard Operating Procedures
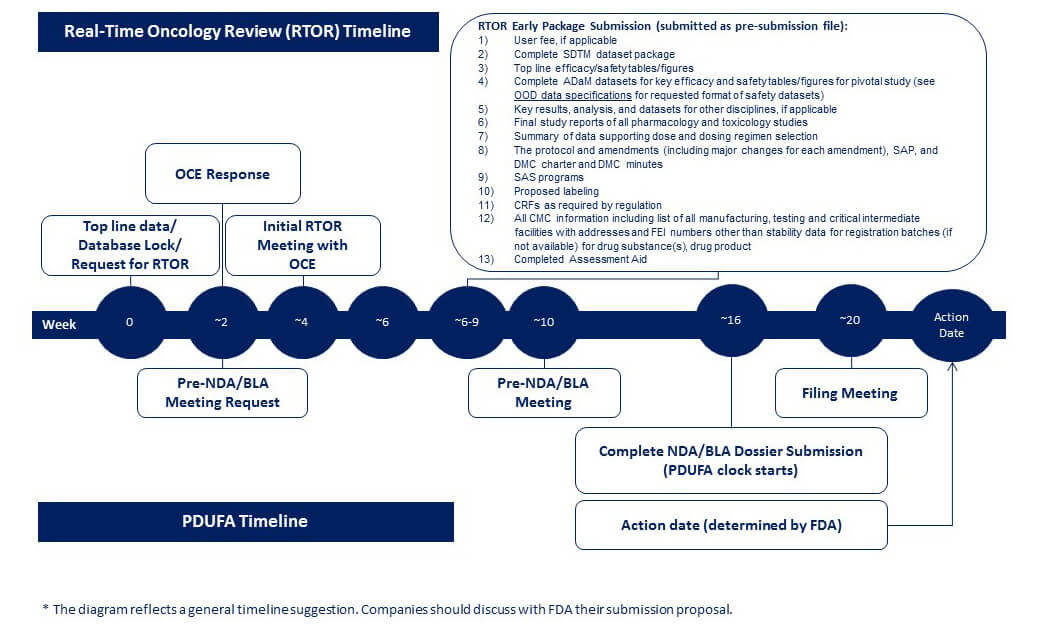
Please note that these milestones are provisional and may vary substantially across applications. The review team is expected to look at the presubmission materials when they are submitted and to discuss their findings with the CDTL and Division Director.
- Week 0-3: At the time of top-line results of a pivotal trial, if the eligibility criteria above is met, an applicant can apply for the RTOR by submitting a request via email to the appropriate application RPM. The clinical division director/deputy director, the review team (including reviewers, team leaders, and management from all relevant review disciplines, and CDRH as applicable) and OCE management will jointly decide whether the application can be selected for the RTOR program. This decision will generally be made in approximately 20 business days of the receipt of notification through the appropriate division RPM.
- Week 3-6: Once an application is selected, a teleconference with the applicant will be arranged in approximately 20 business days. The clinical division director/deputy director, the review team, and OCE staff will participate in this meeting. If the drug product is co-developed with a companion diagnostic, the diagnostic partner and CDRH should also be on the teleconference. If the applicant or the Agency determines that RTOR is not appropriate, a routine review procedure will be followed. Otherwise, FDA and the applicant will discuss the plan for RTOR in detail, reach tentative agreement on responsibilities, and proposed pre-submission timelines. The final SAP/protocol should be submitted as soon as possible. If the drug product is co-developed with a companion diagnostic, the applicant should outline timelines with the diagnostic partner and with CDRH.
- Week 6-9: Under the RTOR, the applicant would officially submit the following items to their marketing application as a pre-submission as soon as they become available:
- User fee, if applicable
- Complete SDTM dataset package
- Top line efficacy/safety tables/figures
- Complete ADaM datasets for key efficacy and safety tables/figures for pivotal study (see OOD data specifications for requested format of safety datasets)
- Key results, analysis, and datasets for other disciplines, if applicable.
- Final study reports of all pharmacology and toxicology studies
- Summary of data supporting dose and dosing regimen selection
- The protocol and amendments (a list of major changes for each amendment), SAP, and DMC charter and DMC minutes
- SAS programs
- Proposed labeling
- CRFs as required by regulation
- All CMC information including list of all manufacturing, testing and critical intermediate facilities with addresses and FEI numbers other than stability data for registration batches (if not available) for drug substance(s), drug product.
- Completed AAid.
- Week 10-16: Pre-submission meeting: In addition to responding to the applicant’s questions in the meeting package, FDA may share with the applicant preliminary key review questions or issues and critical analyses needed. If FDA requests additional analyses, the applicant may submit them before or at the time of submission of the marketing application. In some cases, the applicant may submit the requested additional analyses after the marketing application is submitted.
These discussions may be documented in the meeting minutes under the section, “Agreement of a Complete Application” for NMEs or original BLAs, under a new “Additional Items discussed” which can be added by the RPM for other applications or under specific questions as appropriate.
- Week 16-22: The applicant submits the complete marketing application. Once FDA receives the completed application, the review clock will start. The complete application will include any remaining components previously not submitted in the presubmission.
Figure 1. Proposal for Marketing Application Timeframe of Real-Time Oncology Review (RTOR)
RTOR Frequently Asked Questions
1. What are the main differences between this and how the FDA currently treats NDAs and BLAs?
RTOR allows the FDA to review much of the data earlier, before the applicant formally submits the complete application. First, the applicant will present topline data for the FDA to determine whether RTOR would be appropriate for the application. If the agency determines RTOR is an appropriate review plan, the applicant can start sending pre-submission data to the agency, under the original NDA/BLA, 2-4 weeks after all patient data has been entered and locked by the applicant in their database and the applicant decides to request FDA approval. This pre-submission package should include key raw and derived (ADaM) datasets, including safety and efficacy tables and figures, the study protocol and amendments, and a draft of the prescribing information. In addition, the applicant should also submit key results, analysis, and datasets for other disciplines (e.g., clinical pharmacology), if applicable. The FDA will start evaluating the pre-submitted data for sufficiency and integrity. This informed pre-analysis gives FDA reviewers and applicants an early opportunity to address data quality and potential review issues. The FDA can provide early feedback to the applicant regarding the most effective way to analyze data to properly address key regulatory questions. By the time the applicant submits the complete application to the FDA, the agency’s review team has completed the analysis and is in a better position to conduct a more efficient review. This is different from rolling review, where only fully completed modules can be submitted, and not individual components of a module.
2. If not discussed at a Pre-NDA or BLA meeting, how and where should requests for participation in RTOR be submitted?
If interested in participating in this program, we advise the sponsor to submit an RTOR participation request email to the application RPM (and officially to the IND or marketing application). The sponsor should include a written justification on why their product should be considered for the RTOR program and include top-line results as well as proposed timeline of when they will submit the various components of the application.
3. How will we be notified of acceptance/rejection?
For requests that are not discussed at a PreNDA or BLA meeting, a decision will generally be made in approximately 20 business days of the receipt of notification through the appropriate application RPM. The agency will schedule a teleconference between the sponsor and FDA review team to discuss our decision.
4. If accepted into the RTOR program, when are user fees due?
For supplemental applications, no fees are necessary. For new molecular entities, PDUFA fees (when applicable) will be due when the first component of the RTOR is submitted.
5. Does this impact PDUFA timelines?
PDUFA timelines will not be affected.
6. Does the division need to acknowledge the receipt of pre-submission RTOR submissions?
No. However, if the applicant requests a confirmation of receipt then the application RPM can send a courtesy email confirming receipt.
7. What about companion diagnostic products?
Eligibility of applications with a companion diagnostic for RTOR will be determined on a case by case basis.