FDA Releases Annual Summary Report on Antimicrobials Sold or Distributed in 2018 for Use in Food-Producing Animals
December 10, 2019
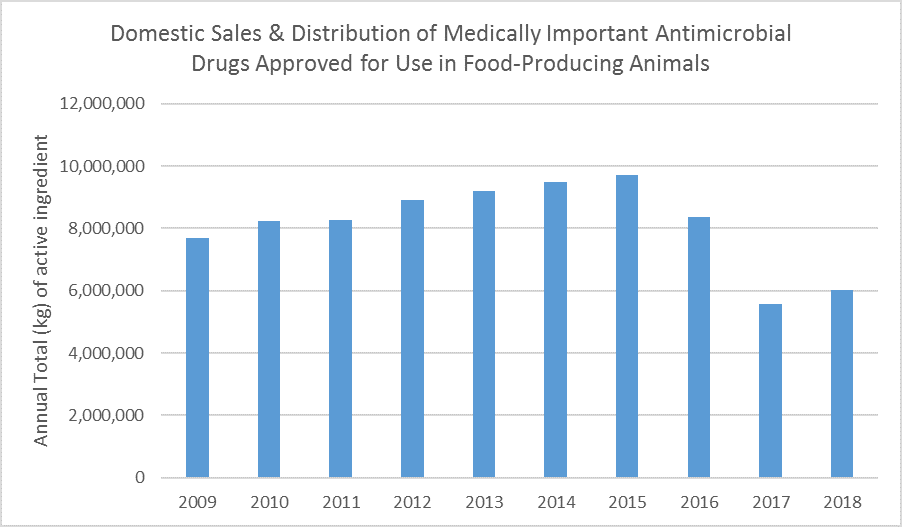
Today the U.S. Food and Drug Administration released the 2018 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals, which showed that domestic sales and distribution of medically important antimicrobials for use in food-producing animals increased nine percent between 2017 and 2018. Despite this increase, 2018 is the second-lowest year on record and the overall trend continues to indicate that ongoing efforts to support antimicrobial stewardship are having an impact: sales in 2018 are down 21 percent since 2009, the first year of reporting, and down 38 percent since 2015, the peak year of sales and distribution.
While sales data do not necessarily reflect actual antimicrobial use, sales volume observed over time is a valuable indicator of market changes related to antimicrobial drug products intended for food-producing animals. However, when evaluating the progress of ongoing efforts to support judicious use of antimicrobials, it is important to take into account additional information sources including actual use data, animal demographics, animal health data, and data on resistance.
FDA’s objective is to slow the development of antimicrobial resistance and preserve the effectiveness of antimicrobials for fighting disease in animals and humans. The agency’s aim is not simply measured by a reduction in sales volume of antimicrobials, but also includes fostering good antimicrobial stewardship practices by optimizing the use of these products and limiting their use in animals to only when necessary to treat, control, or prevent disease.
FDA recognizes that fluctuations in sales volume may occur over time in response to various factors, including changing animal health needs or changes in animal populations. Given the substantial change that occurred with transitioning a large number of products containing medically important antimicrobials from over-the-counter use to a marketing status requiring veterinary oversight at the beginning of 2017, some rebound in the reported sales volume for 2018 was not unexpected as affected stakeholders adjusted to the new requirements.
When analyzing the report, readers should consider:
- Sales and distribution information does not represent actual use of the products.
It is important to acknowledge that these data are sponsor estimates of product sales and are not intended to be a substitute for actual usage data. For example, veterinarians and animal producers may purchase drugs, but never actually administer them to animals, or they may administer the drugs in later years. - Before making a direct comparison between the quantity of antimicrobial drugs sold for use in humans and that sold for use in animals, you should consider:
- There are many more animals than humans. For instance, there are approximately 327 million people in the U.S., while USDA records indicate that about 9.1 billion chickens are slaughtered annually.
- There are differences in physiology and weight between humans and animals: the average adult human weighs 184 lbs., while a beef steer weighs about 1,352 lbs.
- Different animal species metabolize drugs differently, meaning that some may require more of the drug to be effective, or may need to be treated for a longer period of time.
- The agency cautions against making direct comparisons of species-specific sales estimates.
There are a variety of factors that confound direct comparison of species-specific sales estimates, including differences in the population size, weight, lifespan, and drug metabolism of each species. - The agency cautions against making direct comparisons between the sales volume of different drug classes.
Direct comparison of sales volumes for different drug classes is problematic because not all drug classes are approved for use in all species, not all drug potencies are the same, and not all of the drug classes can be used interchangeably to treat the same conditions.
While there has been progress made toward antimicrobial stewardship goals, additional work is needed to address antimicrobial resistance. The Center for Veterinary Medicine’s five-year action plan outlines steps the agency is pursuing to further foster antimicrobial stewardship in veterinary settings.
Additional Information
- 2018 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals
- Questions and Answers: Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals
- ADUFA Reports
- Final Rule to Collect Antimicrobial Sales and Distribution Information by Animal Species
Issued by FDA Center for Veterinary Medicine.
For questions, Contact CVM.