Vet-LIRN Cooperative Agreements
Currently Funded Opportunities
Vet-LIRN Network Capacity-Building Projects (U18)
The cooperative agreement is intended to build domestic laboratory capacity under the Food Safety and Modernization Act (FSMA) and develop Vet-LIRN laboratory capability and capacity to investigate potential animal foodborne illness outbreaks. Vet-LIRN Laboratories may apply for funding for research related to emerging public food safety issues identified by the Vet-LIRN Program Office and for equipment and personnel necessary to expand laboratory capability and capacity. To date, 26 grants were awarded for specific projects and 14 grants were awarded for equipment. In 2021, a Notice of Special Interest was added to this announcement to request applications related to COVID animal diagnostic work.
CVM Vet-LIRN Veterinary Diagnostic Laboratory Program (U18)
Infrastructure funding is key to making sure that the network can function and that laboratories can work to support Vet-LIRN’s mission. As of 2021, 39 labs receive funding from intrastructure grants. These awards facilitate participation in Vet-LIRN activities such as case investigations, emergency exercises, proficiency tests, and laboratory accreditation. The agreements also increase the agency’s capability to analyze an increased number of samples in the event of animal food- or drug-related illnesses or other large-scale emergency events that require increased testing of implicated diagnostic or animal food samples. The new agreement allows network laboratories to request additional funds if they are participating in a specific Vet-LIRN project, such as the Antimicrobial Resistance (AMR) Pilot Project or if they are conducting whole-genome sequencing (WGS) work, or if their caseload is particularly heavy. Additional funding may also be provided for responding to emerging diseases, such as COVID-19.
Previously Funded Opportunities
CVM Vet-LIRN Cooperative Agreement Program to Expand and Validate Testing Methods for Food Contaminants in Animal Diagnostic Specimens (U18) (2013)
Many veterinary diagnostic laboratories use tests that are only validated in their own laboratory. This Cooperative Agreement Program, also called Method Grants, enables the expansion and validation of detection methods that can be used by network laboratories, thereby increasing the suite of validated methods available for testing during case investigations, outbreaks, or other emergency events. Testing animal diagnostic specimens enables Vet-LIRN to investigate consumer complaints about animal food or drugs. Such investigations require validated test methods for animal diagnostic samples, such as urine, blood, feces, saliva, liver, and kidney; these are not typical food matrices, and traditional food-testing laboratories do not test for them. The program also strengthens communication, collaboration, and integration of the network laboratories, which is crucial for providing a quick response in an emergency. Seven laboratories received funding for chemistry-related projects and three received funding for surveillance and sequencing of select pathogens in diagnostic samples.
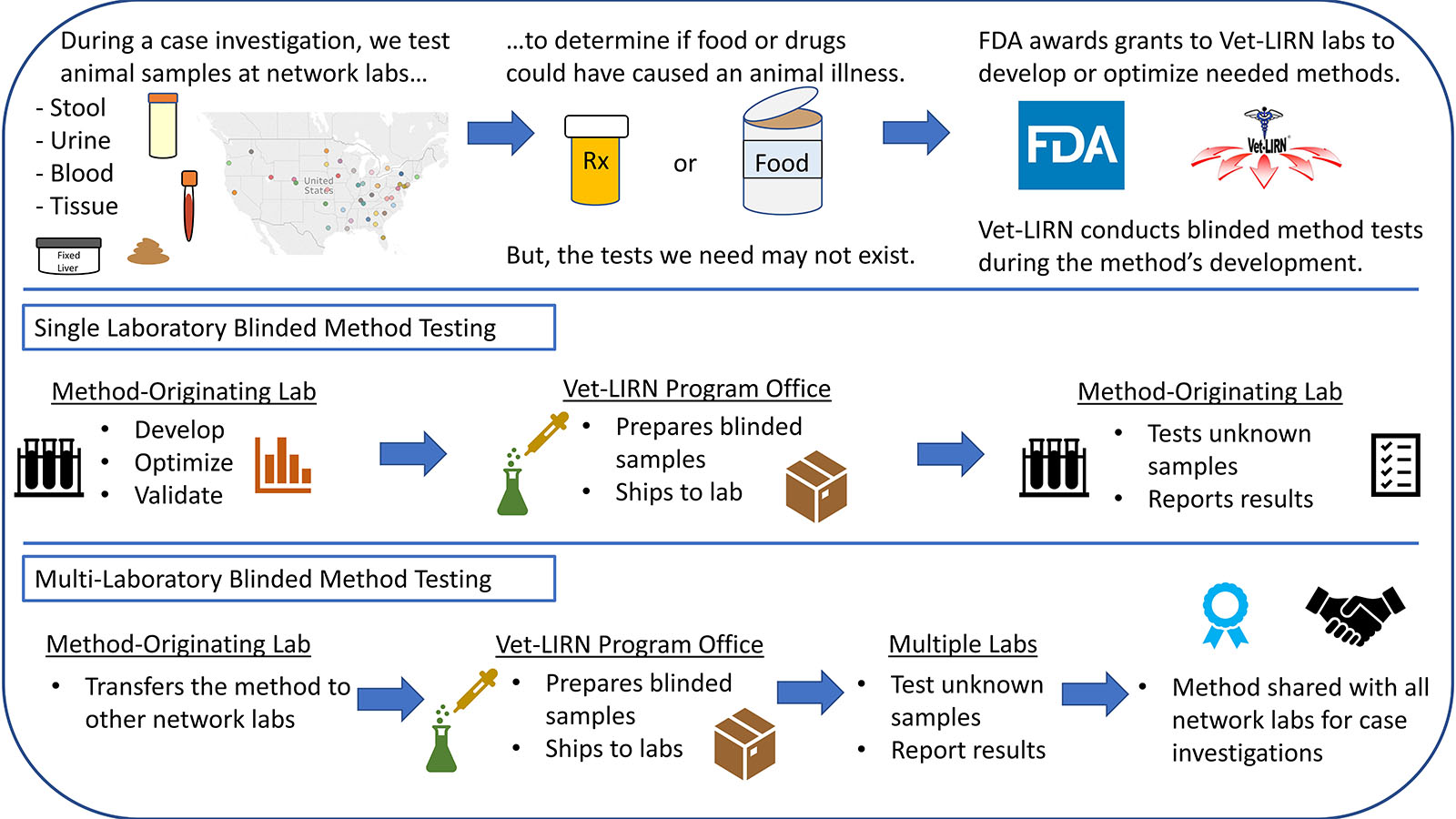
Figure 1. What are the steps during a Blinded Method Test?
Blinded Method Test
Testing animal diagnostic specimens enables Vet-LIRN to investigate consumer complaints about animal food or drugs. The Methods and Capacity-Building Grants help Vet-LIRN ensure that the data from our laboratories is derived from methods that have been validated. Although Vet-LIRN methods are not regulatory methods, the FDA Method Validation Guidelines are generally followed during the method development and evaluation. Most of the projects have the following phases:
- Method-Originating Laboratory (MO) – Cooperative-Grant Recipient
- Method development/optimization.
- Validation by the single MO laboratory.
- Vet-LIRN Program Office
- Vet-LIRN evaluates the method performance in the MO laboratory through exercises called Blinded Method Tests (BMTs). Vet-LIRN prepares samples, and the MO laboratory analyzes blinded samples.
- MO Laboratory
- Method transfer to other Vet-LIRN collaborating laboratories.
- Vet-LIRN Program Office
- Vet-LIRN evaluates the method performance in multiple laboratories with a Blinded Method Test.
The methods developed by our Vet-LIRN laboratories are extensively evaluated and will be
- published in a scientific journal, and
- adopted and used by Vet-LIRN laboratories for animal diagnostic purposes.
Highlighted methods that completed BMTs in 2019 include
- University of Kentucky – Evaluation of LC-MS/MS methods to detect multiple anticoagulant rodenticides in liver and blood
Anticoagulant rodenticide poisonings of non-target species (i.e., wildlife, domestic farm animals, and pets) due to accidental consumption and by malicious adulteration (baiting) occur and can be confused with food-related toxicities by owners of the animals, thus a reliable method is needed to detect the chemicals in animal samples. - University of Pennsylvania – Evaluation of CHARM based method to detect aflatoxin B1 in various dog foods
Mold can produce aflatoxin B1 in food ingredients such as grains (e.g. corn, wheat, barley), rice, nuts and fruits. Aflatoxin B1 is the most toxic aflatoxin and is known for its mutagenic, carcinogenic, teratogenic, and immunosuppressive impact. It can cause death or intoxication in livestock and pets resulting in reduced growth rate, immunosuppression, and liver injury. Therefore, fast methods to identify this toxicant in animal food are desirable. - University of California, Davis – Evaluation of methods to detect multiple carbamate pesticides in rumen contents
Accidental pesticide poisonings can be mistaken for foodborne toxicities, so there is a need to identify these compounds in tissues or stomach contents. The new method identifies 12 pesticides at once in stomach contents of ruminants. - Iowa State University – Development and evaluation of methods to detect aflatoxins in complex matrices such as liver and urine
Many species of mold can produce aflatoxins B1, B2, G1, and G2 in a wide variety of food ingredients such as corn, barley, rice, nuts, and fruits. Aflatoxins can also undergo chemical modifications (into M1, Q1) in animal liver after being consumed. Since representative feed samples may not be available and feed-based test methods are not confirmatory of an etiologic diagnosis, there is a need for a method to detect these chemicals in animal tissues. A urine test will allow diagnosis to be made in living animals.
Evaluation of Salmonella in Symptomatic and Asymptomatic Pets - a Vet-LIRN Program Cooperative Agreement
From 2012 to 2014, eleven Vet-LIRN cooperative-agreement laboratories evaluated Salmonella prevalence in the cat and dog pet population across the country. This study helps CVM to understand the estimated prevalence of Salmonella in the dog and cat population. The harmonized method ensures consistent data is obtained from Vet-LIRN veterinary diagnostic laboratories participating in this special investigation. Data obtained from this study identified various serotypes of Salmonella found in household pets and will provide a baseline of data that can be used in subsequent outbreak investigations. Over 3,000 samples were tested. Laboratories collected samples from dogs and cats without signs of salmonellosis (asymptomatic) and with signs of potential salmonellosis (symptomatic). Of the positive cases, approximately half of the dogs were non-diarrheic. Salmonella-positive dogs were more likely to have consumed raw food or to have consumed probiotics. In addition to our initial plan, we conducted whole-genome sequencing (WGS), pulsed-field gel electrophoresis (PFGE) and antimicrobial susceptibility testing (AST) on all isolates. The work was published in the Journal of Clinical Microbiology.
Publications
Vudathala, D., et al., Multilaboratory Evaluation of a Lateral Flow Method for Aflatoxin B1 Analysis in Dry Dog Food. J AOAC Int, 2020. 103(2): p. 480-488.
Du, X., et al., Evaluation of a Diagnostic Method to Quantify Aflatoxins B(1) and M(1) in Animal Liver by High-Performance Liquid Chromatography with Fluorescence Detection. J AOAC Int, 2019. 102(5): p. 1530-1534.
Du, X., et al., Intra-laboratory Development and Evaluation of a Quantitative Method for Measurement of Aflatoxins B1, M1 and Q1 in Animal Urine by High Performance Liquid Chromatography with Fluorescence Detection. Journal of Analytical Toxicology, 2017. 41(8): p. 698-707.
Smith, L.L., et al., Development and Validation of Quantitative Ultraperformance Liquid Chromatography-Tandem Mass Spectrometry Assay for Anticoagulant Rodenticides in Liver. J Agric Food Chem, 2017. 65(31): p. 6682-6691.
Goodman, L.B., et al., Detection of Salmonella spp. in veterinary samples by combining selective enrichment and real-time PCR. 2017. 29(6): p. 844-851.
Leahy, A.M., et al., Faecal Campylobacter shedding among dogs in animal shelters across Texas. Zoonoses Public Health, 2017. 64(8): p. 623-627.
Shao, D., et al., Intralaboratory development and evaluation of a high-performance liquid chromatography-fluorescence method for detection and quantitation of aflatoxins M1, B1, B2, G1, and G2 in animal liver. J Vet Diagn Invest, 2016. 28(6): p. 646-655.
Goodman, L.B., et al., High-throughput Detection of Respiratory Pathogens in Animal Specimens by Nanoscale PCR. Journal of Visualized Experiments : JoVE, 2016(117): p. 54781.