Center for Drug Evaluation and Research Response to Coronavirus (COVID-19) | Infographic
Visualization of data associated with CDER's response to the coronavirus (COVID-19) pandemic from January 1, 2020 – September 30, 2021
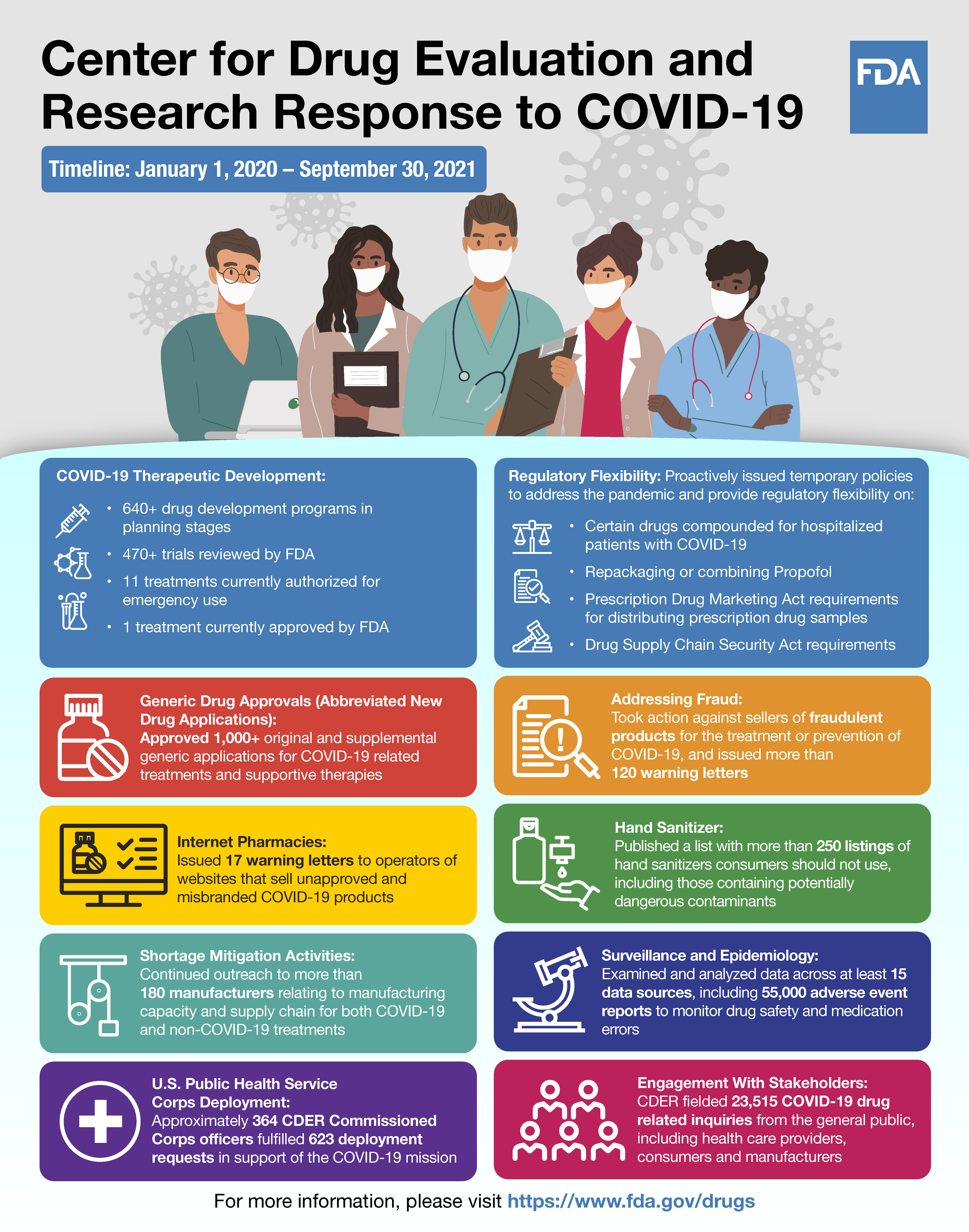
Timeline: January 1, 2020 – September 30, 2021
COVID-19 Therapeutic Development
- 640+ drug development programs in planning stages
- 470+ trials reviewed by FDA
- 11 treatments currently authorized for emergency use
- 1 treatment currently approved by FDA
Regulatory Flexibility
Proactively issued temporary policies to address the pandemic and provide regulatory flexibility on:
- Certain drugs compounded for hospitalized patients with COVID-19
- Repackaging or combining Propofol
- Prescription Drug Marketing Act requirements for distributing prescription drug samples
- Drug Supply Chain Security Act requirements
Generic Drug Approvals (Abbreviated New Drug Applications)
Approved 1,000+ original and supplemental generic applications for COVID-19 related treatments and supportive therapies
Addressing fraud
Took action against sellers of fraudulent products for the treatment or prevention of COVID-19 and issued more than 120 warning letters
Internet pharmacies
Issued 17 warning letters to operators of websites that sell unapproved and misbranded COVID-19 products
Hand sanitizer
Published a list with more than 250 listings of hand sanitizers consumers should not use, including those containing potentially dangerous contaminants
Shortage mitigation activities
Continued outreach to more than 180 manufacturers relating to manufacturing capacity and supply chain for both COVID-19 and non-COVID-19 treatments
Surveillance and Epidemiology
Examined and analyzed data across at least 15 data sources, including 55,000 adverse event reports to monitor drug safety and medication errors
U.S. Public Health Service Corps deployment
Approximately 364 CDER Commissioned Corps officers fulfilled 623 deployment requests in support of the COVID-19 mission
Engagement with stakeholders
CDER fielded 23,515 COVID-19 drug related inquiries from the general public, including health care providers, consumers and manufacturers
For more information, please visit www.fda.gov/drugs