Prescription Drug Labeling Resources
FDA's Prescription Drug Labeling Resources website provides over 150 labeling resources for the Prescribing Information, FDA-approved patient labeling, and/or carton and container labeling for human prescription drugs, including biological products (including over 50 guidances with labeling content) - see Overview of Website.
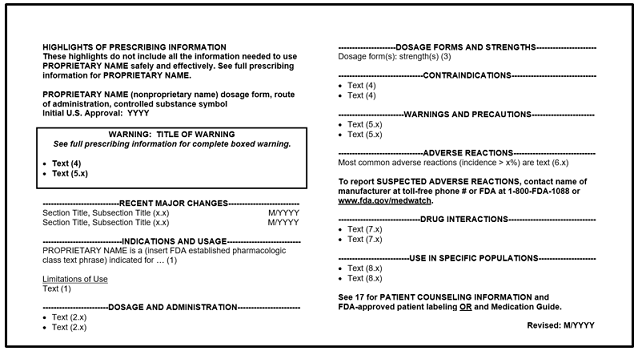
Highlights of Prescribing Information: Format Sample
Table of Contents
- Overview of Website
- Prescribing Information and Application Holder Responsibilities
- Prescribing Information Requirements and Rules
- Broad Labeling Resources
- Indications and Usage, Dosage and Administration, and Clinical Studies Information in Labeling
- Pregnancy, Lactation, and Females and Males of Reproductive Potential Information in Labeling
- Pediatric, Geriatric, and Other Specific Population Information in Labeling
- Safety-Related Information in Labeling
- Clinical Pharmacology Information in Labeling
- Microbiology Information in Labeling
- Sample Templates and Format Tools for Prescribing Information
- Established Pharmacologic Class (EPC) Resources
- Generic Drug Products -Specific Labeling Resources
- Biological Product-Specific Labeling Resources
- Product Quality-Related Labeling Resources
- Carton and Container Labeling Specific Resources
- Patient Labeling Specific Resources
- Structured Product Labeling Resources
- Labeling-Related Databases
- Additional Labeling Resources
- Articles with Labeling Content
- Healthcare Practitioner Labeling Resources
- Questions
Overview of Website
FDA’s Prescription Drug Labeling Resources website [formerly known as the PLR Requirements for Prescribing Information website] provides resources for the development of human prescription drug, including biological product, labeling regulated under New Drug Applications (NDAs), Biologics License Applications (BLAs), and Abbreviated New Drug Applications (ANDAs).
- Labeling for such products includes but is not limited to:
- Prescribing Information (PI)
- FDA-approved patient labeling [Medication Guides, Instructions for Use, and Patient Information (also called Patient Package Inserts)], and
- Carton and container labeling.
- The PI has two formats: “Physician Labeling Rule” (PLR) format and “old” (non-PLR) format). Given that all new human prescription drugs, including biological products, approved since June 2001 and certain new human prescription drugs, including biological products, approved before June 2001 (e.g., those approved for new uses after June 2001) must have PI in PLR format, this website focuses on providing resources for the development of PI with PLR format labeling.
- We strongly encourage application holders to voluntarily convert PI approved in “old” format to PLR format because we believe that PLR format labeling enhances the safe and effective use of human prescription drugs, including biological products, and reduces the number of adverse reactions resulting from medication errors due to misunderstood or incorrectly applied drug information.
This website includes the following PI resources: labeling regulations, guidances, presentations, templates, format tools, databases, and additional labeling resources. This website also includes resources for FDA-approved patient labeling (Instructions for Use, Medication Guides, and Patient Information) and carton and container labeling. Not every resource will be applicable to every prescription drug or biological product.
This website does not include:
- Promotional labeling resources for human prescription drugs, including biological products.
- Labeling resources for other FDA-regulated products such as nonprescription drug products ("over-the-counter" drugs), devices, homeopathic products, dietary supplements, foods, tobacco, or animal drugs.
Prescribing Information and Application Holder Responsibilities
The Prescribing Information is written for the healthcare practitioner and must:
- Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological product.
- Be informative and accurate and neither promotional in tone nor false or misleading
- Be updated when new information becomes available that causes labeling to become inaccurate, false, or misleading
Application holders should review PI at least annually for outdated information.
Prescribing Information Requirements and Rules
- Physician Labeling Rule: This January 2006 final rule revised the content and format of PI for human prescription drugs, including biological products.
- 21 CFR 201.56: General requirements on the content and format of PLR format labeling and “old” format (non-PLR) labeling
- 21 CFR 201.57: Specific requirements on content and format of PLR format labeling for human prescription drugs, including biological products
- Pregnancy and Lactation Labeling Rule: This December 2014 final rule revised the content and format of pregnancy, lactation, and females and males of reproductive potential information in labeling
- 21 CFR 201.80: Specific requirements on content and format of “old” format (non-PLR) labeling for human prescription drugs, including biological products
Broad Labeling Resources
- Implementing the PLR Content and Format Requirements (final guidance)
- Voluntary PLR Conversions and Updating Prescribing Information (2019 presentation and video)
- Improving Consistency of Information Between Carton-Container Labeling and Prescribing Information (2019 presentation and video)
- Drugs@FDA vs. DailyMed, Labeling Resources, and Future Labeling Guidances (2019 presentation)
- CDER’s Review of the Prescribing Information (2019 presentation)
- Updating Prescription Drug and Biological Product Labeling (2018 presentation)
- Consistency in Labeling and Methods to Optimize Communication in Labeling (2017 presentation)
- Converting Labeling for Older Drugs from Old Format to PLR Format (2017 presentation) (video)
- PLR Implementation, CDER Staff for Labeling Review, and Labeling Resources (2017 presentation) (video)
- CDER's Review of Prescribing Information (2017 presentation)
- Prescribing Information - Resources and Review Process (2016 presentation)
- Highlights of Prescribing Information (2015 presentation)
- Prescribing Information Potpourri (2015 presentation)
Indications and Usage, Dosage and Administration, and Clinical Studies Information in Labeling
- Indications and Usage Section of Labeling (draft guidance)
- Indications and Usage Section of Labeling Draft Guidance (2019 presentation and video)
- Considerations for Developing Indications and Usage Section (2017 presentation) (video)
- Indications and Usage Section (2015 presentation)
- Labeling Case Study - Transformation of an Indication (2019 presentation and video)
- Labeling for Human Prescription Drug and Biological Products Approved Under the Accelerated Approval Regulatory Pathway (final guidance)
- Limited Population Pathway for Antibacterial and Antifungal Drugs (final guidance) NEW!
- Dosage and Administration Section of Labeling (final guidance)
- Dosage and Administration Section (2015 presentation)
- Clinical Studies Section of Labeling (final guidance)
- Considerations for the Clinical Studies Section of Labeling (2017 presentation)
- Clinical Studies Section (2015 presentation)
Pregnancy, Lactation, and Females and Males of Reproductive Potential Information in Labeling
- Pregnancy and Lactation Labeling Rule (PLLR): This December 2014 final rule revised the content and format of pregnancy, lactation, and females and males of reproductive potential information in labeling
- Pregnancy, Lactation, and Reproductive Potential: Labeling for Human Prescription Drug and Biological Products-Content and Format (draft guidance) NEW!
- Oncology Pharmaceuticals: Reproductive Toxicity Testing and Labeling Recommendations (final guidance)
- PLLR: Four Years In – What’s Next? (2019 presentation)
- PLLR: Two Years In – Incorporating Human Pregnancy Data in Labeling (2017 presentation)
- The Pregnancy and Lactation Labeling Rule (2016 presentation)
- PLLR implementation schedule
- Additional PLLR Resources: Labeling for use of prescription drugs, including biological products, during pregnancy, during lactation, and in females and males of reproductive potential
Pediatric, Geriatric, and Other Specific Population Information in Labeling
- Pediatric Information Incorporated Into Human Prescription Drug and Biological Products Labeling (final guidance)
- Pediatric Information in Labeling (2019 presentation and video)
- Geriatric Information in Human Prescription Drug and Biological Product Labeling (draft guidance) NEW!
- Promoting Safe and Effective Prescription Drug Use in Geriatric Patients (2020 presentation and video) NEW!
- Distributing Specific Population Information in Labeling (2015 presentation)
Safety-Related Information in Labeling
- Warnings and Precautions, Contraindications, and Boxed Warning Sections of Labeling (final guidance)
- Adverse Reactions Section of Labeling (final guidance)
- Adverse Reaction Information in Labeling (2019 presentation and video)
- Safety-Related Information in the Prescribing Information (2015 presentation)
- Drug Abuse and Dependence Section of Labeling (draft guidance)
- Drug Abuse and Dependence Section of Labeling Draft Guidance (2019 presentation and video)
- Abuse-Deterrent Opioids - Evaluation and Labeling (final guidance)
- Best Practices in Developing Proprietary Names for Drugs (draft guidance)
- Safety Labeling Changes - Implementation of Section 505(o)(4) of the FDC Act (final guidance)
- Safety Labeling Changes Under Section 505(o)(4) of the FD&C Act (MAPP)
- Public Availability of Labeling Changes in “Changes Being Effected” Supplements (draft guidance)
- Drug Safety-Related Labeling Changes Database - provides recent updates on safety information in labeling. This database includes labeling changes (1) from labeling and efficacy supplement approvals and (2) required by the FDA under Section 505(o)(4) of the FD&C Act (safety labeling changes).
- Drug Safety Communications – contains the most recent Drug Safety Communications from FDA
Clinical Pharmacology Information in Labeling
- Labeling Made Simple: The How, What, and Where of Drug Interactions in Prescribing Information (2020 presentation and video).
- Describing Clinically Significant Drug Interactions in the Warnings and Precautions Section (2015 presentation)
- Clinical Drug Interaction Studies - Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions (final guidance) NEW!
- Clinical Pharmacology Section of Labeling (final guidance)
- A Recipe for Clinical Pharmacology Information in Labeling (2019 presentation and video)
- The Ins and Outs of Presenting Clinical Pharmacology Information in Prescription Drug Labeling (2017 webinar)
- Cracking the Code for Clinical Pharmacology-Related Prescription Drug Labeling (2017 presentation) (video)
- Strategies for Enhancing Quality, Utility, & Clarity in Clinical Pharmacology-Related Labeling (2017 presentation)
- Assessing the Effects of Food on Drugs in INDs and NDAs – Clinical Pharmacology Considerations (draft guidance)
- Pharmacokinetics in Patients with Impaired Renal Function - Study Design, Data Analysis, and Impact on Dosing (draft guidance) NEW!
- Clinical Pharmacogenomics: Premarket Evaluation in Early-Phase Clinical Studies and Recommendations for Labeling (final guidance)
Microbiology Information in Labeling
- Limited Population Pathway for Antibacterial and Antifungal Drugs (final guidance) NEW!
- Microbiology Data for Systemic Antibacterial Drugs - Development, Analysis, and Presentation (final guidance)
- Systemic Antibacterial and Antifungal Drugs: Susceptibility Test Interpretive Criteria Labeling for NDAs and ANDAs (final guidance)
Sample Templates and Format Tools for Prescribing Information
- Final Labeling Check of Labeling Format and Appearance (2019 presentation and video)
- Recommendations for Final Labeling Format Check Prior to End-of-Cycle Labeling Submission
- Sample PLR Template - Highlights, Contents, and Full Prescribing Information
- Selected Requirements of Prescribing Information (SRPI) - A 41-item, drop-down checklist of important format elements of the PI based on regulations (21 CFR 201.56 and 201.57) and guidances
- SRPI Video - Highlights
- SRPI Video - Table of Contents and Full Prescribing Information
Established Pharmacologic Class (EPC) Resources
- Determining the EPC for Use in Highlights MAPP
- Determining the EPC for Use in Highlights Guidance
- FDA EPC Text Phrases for Indications and Usage heading in Highlights (updated October 2021) NEW!
Generic Drug Products -Specific Labeling Resources
- Orange Book
- Updating ANDA Labeling After the Marketing Application for the RLD Has Been Withdrawn (draft guidance)
- Acceptability of Draft Labeling to Support ANDA Approval (final guidance)
- Referencing Approved Drug Products in ANDA Submissions (final guidance)
- Determining Whether to Submit an ANDA or a 505(b)(2) Application (final guidance)
- Good ANDA Submission Practices (draft guidance)
Biological Product-Specific Labeling Resources
- Labeling for Biological Products (2019 presentation and video)
- Labeling for Biosimilar Products (final guidance)
- Labeling for Biosimilar Products (2019 presentation)
- Biosimilar Product Information: Includes links to FDA-approved labeling for licensed biosimilar products.
- Nonproprietary Naming of Biological Products (final guidance)
- Nonproprietary Naming of Biological Products: Update (draft guidance)
- Procedures for Handling Requests for Nonproprietary Name Suffix Review for Biological Products Newly Licensed Under Section 351of the PHS Act MAPP
- The “Deemed to be a License” Provision of the BPCI Act - Questions and Answers (final guidance) NEW!
- Products That Were Deemed to be BLAs on March 23, 2020 NEW!
- The Purple Book
Product Quality-Related Labeling Resources
- Considerations for Product Quality Information in Prescribing Information (2017 presentation)
- Product Title and Initial U.S. Approval in the Highlights of Prescribing Information (2019 presentation and video)
- Drug Product Nomenclature (2019 presentation and video)
- Product Title and Initial U.S. Approval in the Highlights of Prescribing Information (draft guidance)
- Example Product Titles in Highlights of Prescribing Information NEW!
- Selection of the Appropriate Package Type Terms and Recommendations for Labeling Injectable Medical Products Packaged in Multiple-Dose, Single-Dose, and Single-Patient-Use Containers for Human Use (final guidance)
- Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation (final guidance)
- Naming of Drug Products Containing Salt Drug Substances (final guidance)
- Naming of Drug Products Containing Salt Drug Substances MAPP
- Quality Attribute Considerations for Chewable Tablets (final guidance)
- Recommendations for Labeling Medical Products to Inform Users that the Product or Product Container is not Made with Natural Rubber Latex (final guidance)
- Child-Resistant Packaging Statements in Drug Product Labeling (final guidance)
- Gluten in Drug Products and Associated Labeling Recommendations (draft guidance)
- Liposome Drug Products: Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation (final guidance)
- Metered Dose Inhaler (MDI) and Dry Powder Inhaler (DPI) Drug Products--Quality Considerations (draft guidance)
- Use of Liquids and/or Soft Foods as Vehicles for Drug Administration: General Considerations for Selection and In Vitro Methods for Product Quality Assessments (draft guidance)
- Best Practices in Developing Proprietary Names for Drugs (draft guidance)
- Incorporation of Physical-Chemical Identifiers into Solid Oral Dosage Form Drug Products for Anticounterfeiting (final guidance)
- Harmonizing Compendial Standards With Drug Application Approval Using the USP Pending Monograph Process (draft guidance)
- Transdermal and Topical Delivery Systems - Product Development and Quality Considerations (draft guidance)
Carton and Container Labeling Specific Resources
- Allowable Excess Volume and Labeled Vial Fill Size in Injectable Drug and Biological Products (final guidance)
- Product Identifiers Under the Drug Supply Chain Security Act Questions and Answers (draft guidance)
- Product Identifier Requirements Under the Drug Supply Chain Security Act – Compliance Policy (final guidance)
- Bar Code Label Requirements Questions and Answers (final guidance)
- Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors (draft guidance)
- Safety Considerations for Carton and Container Labeling (2019 presentation and video)
- Improving Consistency of Information Between Carton-Container Labeling and Prescribing Information (2019 presentation and video)
Patient Labeling Specific Resources
- Instructions for Use - Patient Labeling for Human Prescription Drug and Biological Products and Drug-Device and Biologic-Device Combination Products (draft guidance) - Instructions for Use (IFU) are a type of FDA-approved patient labeling for drugs that have complicated or detailed patient-use instructions. The IFU provides detailed, action-oriented, step-by-step written and visual instructions for the patient on how to use the drug including instructions on preparation, administration, handling, storage, and disposal.
- Instructions for Use Draft Guidance (2019 presentation and video)
- Medication Guides: Medication Guides are a type of FDA-approved patient labeling for drugs used primarily on an outpatient basis when the FDA determines that it is necessary for patient’s safe and effective use.
- 21 CFR 208: Medication Guide regulations
- Patient Package Inserts (PPIs) are a type of FDA-approved patient labeling that are required for oral contraceptives (21 CFR 310.501) and estrogen-containing products (21 CFR 310.515). PPIs are voluntary for other prescription drug products.
Structured Product Labeling Resources
- Logical Observation Identifiers Names and Codes (LOINC) for Human Prescription Drug and Biological Product Labeling: For human prescription drugs, including biological products, this document links LOINC codes and names to (1) sections and subsections of PLR and “old” format labeling, (2) types of FDA-approved patient labeling, and (3) the principal display panel
- Improving the Accuracy of Structured Product Labeling (SPL) Submissions – The Missing LOINC (2019 presentation and video)
- Electronic Drug Registration and Listing Instructions
- Additional SPL Resources: SPL is the standard format for electronic submission of the content of labeling
Labeling-Related Databases
- Drugs@FDA - includes information about drugs, including biological products, approved for human use in the United States (e.g., product information, regulatory history, most recent FDA-approved Prescribing Information and patient labeling, and reviews by FDA staff that evaluate the safety and effectiveness of the product). Drugs@FDA does not include information about FDA-approved products regulated by the Center for Biologics Evaluation and Research or products not approved by the FDA.
- DailyMed - NIH’s labeling search tool over 130,000 labeling documents for prescription drugs (including biological products, vaccines, blood products, cellular and gene therapy products), over-the-counter drugs, homeopathic drugs, animal drugs, and other products.
- FDALabel - FDA’s labeling search tool over 130,000 labeling documents. FDALabel and DailyMed have the same database but have different search functions and different displays of search results.
- Pediatric Labeling Information Database - highlights important labeling changes related to pediatric use.
- Drug Safety-Related Labeling Changes Database - provides recent updates on safety information in labeling. This database includes labeling changes (1) from labeling and efficacy supplement approvals and (2) required by the FDA under Section 505(o)(4) of the FD&C Act (safety labeling changes).
- National Drug Code (NDC) Directory – search for NDC codes
- Medication Guides
- Orange Book
- Products That Were Deemed to be BLAs on March 23, 2020
- Biosimilar Product Information
- The Purple Book
- CDER’s Novel Drugs and Biological Products
- CBER’s Novel Biological Products
Additional Labeling Resources
- Patient Counseling Information Section of Labeling (final guidance)
- Patient Counseling Information Section (2015 presentation)
- Medical Product Communications That Are Consistent With the FDA-Required Labeling - Questions and Answers (final guidance)
- Importation of Certain FDA-Approved Human Prescription Drugs, Including Biological Products, under Section 801(d)(1)(B) of the Federal FD&C Act (final guidance)
- Importation of Prescription Drugs (final rule) - 85 FR 62094
- CDER Manual of Policies and Procedures (MAPP): MAPPs establish a system for issuing directives in the Center for Evaluation and Research (CDER) to document and disseminate CDER policies and procedures.
- FDA Guidances: Refer to this page for other guidances that contain labeling recommendations and product-specific guidances.
- Labeling Policy Team in the Office of New Drugs at the FDA
Articles with Labeling Content
- Gassman, A. L, Nguyen, C. P., and Joffe, H. V. (2017). FDA Regulation of Prescription Drugs. The New England Journal of Medicine, 376: 674-82. https://www.nejm.org/doi/10.1056/NEJMra1602972
- Balogh EP, Bindman AB, Eckhardt SG, Halabi S, Harvey RD, Jaiyesimi I, Miksad R, Moses HL, Nass SJ, Schilsky RL, Sun S, Torrente JM, and Warren KE. (2019). Challenges and Opportunities to Updating Prescribing Information for Longstanding Oncology Drugs. The Oncologist, 24:1–7. https://theoncologist.onlinelibrary.wiley.com/doi/10.1634/theoncologist.2019-0698
- Guinn, D., Madabushi, R., Wang, Y., Brodsky, E., Zineh, I., and Maxfield, K. Communicating Immunogenicity-Associated Risk in Current U.S. FDA Prescription Drug Labeling: A Systematic Evaluation. Ther Innov Regul Sci (2020). https://doi.org/10.1007/s43441-020-00161-z
Healthcare Practitioner Labeling Resources
- Labeling Made Simple: The How, What, and Where of Drug Interactions in Prescribing Information (2020 presentation and video).
- Labeling on Drugs@FDA vs. DailyMed (2017 presentation)
- Professional Labeling: The Prescribing Information (2015 presentation)
Questions
For general questions about the Prescribing Information or this website see the Labeling Policy Team webpage and for specific questions about labeling under an NDA, BLA, or ANDA please contact the regulatory project manager assigned to the application.