Impact Story: Helping to Better Communicate the Risks and Benefits in Prescription Drug Advertising
FDA is leading research to understand how images, music, and other elements of direct-to-consumer advertising affect consumer understanding and retention of the risks and benefits of prescription drugs.
Direct-to consumer (DTC) advertisements are a major vehicle for drug companies to promote prescription drugs to the public. Appearing in general-audience magazines, in newspapers, on television, or on the radio, these ads have an influence on consumers' awareness of medications, motivation to seek out medications, and possible use of prescription drugs. As part of FDA's regulatory authority, the Office of Prescription Drug Promotion (OPDP), within CDER, is charged with making sure drug sponsors are in compliance with the specific regulations that govern promotional content to ensure that these ads are truthful and balanced.
CDER social scientists study how to improve the advertisements to enhance the public's understanding of the risks and benefits of prescription drug products. This research includes surveys of consumer experience of DTC advertising and other kinds of observational studies, as well as studies using random assignment. These studies allow investigators to assess the effects of specific changes in the organization, format, and content (audio and visual) of the ads on consumer memory, understanding, interpretation, and retention of critical information. Drug companies can then use this research to develop advertisements that effectively convey important information about their products.
The Scientific Challenge
DTC ads on television are often a complex combination of visual elements (e.g., changing scenes from the life of a patient who has a serious condition), music, voiceover narration about potential benefits and risks, and written text. Research is needed to better understand how these elements influence consumer understanding and perceptions.
FDA's Research on Retention and Perception of Risk Information in DTC Advertising
In a recent study, CDER researchers sought to understand how distracting elements in ads influence viewers' attention to the risk statement, retention of the risks conveyed in an ad, and overall perception of risk.
Study Design:
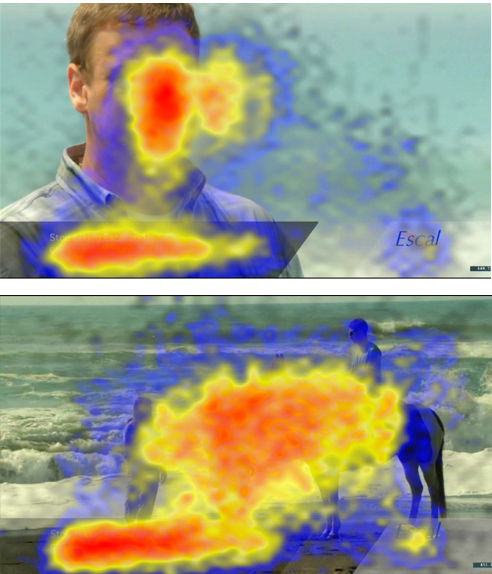
Figure 1. Viewers' focus shifts away from the risk statement in a high-distraction ad.
CDER researchers used eye tracking technology to measure how long study participants' eyes were focused on specific locations in two model television advertisements for a drug. The blackened area in the lower left of each ad shown included the text of the drug's risk statement. In the low-distraction ad shown at the top, a man narrates the risk statement while the risk text is displayed. Participants' eyes focused on the narrator and the risk statement (red indicates longer focus on a given area). In the high-distraction ad shown at the bottom, the risk statement was accompanied by rapidly changing scenes of people engaged in various recreational activities. The visual focus of participants who viewed this ad tended to be diverted away from the risk statement, and retention of the risk information was decreased.
The researchers created two versions of a fictional drug ad in which the risks were identical.
- In the low-distraction ad version, an actor was shown reading the risk statement aloud while the risk statement was shown in text on the screen.[1]
- In the high-distraction ad, the risks were presented in the audio as a voiceover and in text on the screen, while the visuals showed a rapid succession of scenes of people engaged in recreational activities.
The researchers randomly assigned 300 research participants to view one of the drug ads. Eye tracking technology measured the percentage of time participants' eyes focused on the text of the risk statement.
Participants were then asked to recall and recognize the drug's risks and estimate the likelihood and magnitude of the risk posed by the drug.
Results:
- The researchers found that even though both ads presented the risk information in audio and visual form, participants who viewed the high-distraction ad were less able to recall or subsequently recognize the risks described.
- Participants who viewed the high-distraction ad also spent less time with their eyes focused on the written statement of risks than those who viewed the low-distraction ad.
Statistical analyses indicated that this decreased attention partly explained the lower recall and recognition. Participants' risk perceptions (e.g., how likely a patient taking the drug would experience side effects and the perceived seriousness of those side effects) were not significantly different for the ads used in this study.
The researchers also assessed the effect of including the MedWatch statement,[2] which encourages consumers to report any negative side effects to FDA. The researchers found that attention to the MedWatch statement, as measured by eye tracking, did not affect the participants' ability to recall or recognize the drug's risks.
This study, which provided important evidence on the potential effects of distracting elements, is one of several recently completed projects in which CDER investigated the attributes of DTC ads. Additional studies provided important insights into how the marketing and advertising of prescription drugs affects consumers and health care professionals.
For more information, please visit the Office of Prescription Drug Promotion (OPDP) and read more about OPDP research.
Direct-to-consumer advertising can influence awareness of prescription drug treatments, which can in turn influence which prescription drugs patients seek out and doctors prescribe. FDA researchers identified elements of these ads that can affect the viewer's ability to understand the risks and benefits of a drug, helping drug companies develop ads that better inform the public.
Related Publications
Aikin, KJ, BG Southwell, BG, RS Paquin, et al., 2017, Correction of misleading information in prescription drug television advertising: The roles of advertisement similarity and time delay, Res Soc Admin Pharm, 13(2):378-388.
Aikin, KJ, HW Sullivan HW, AC O'Donoghue and KR Betts, 2016, Consumer perceptions of prescription and over-the-counter drug advertisements with promotional offers, Health Mark Q, 33(4):291-306.
O'Donoghue, AC, HW Sullivan, KJ Aikin, D Chowdhury, RR Moultrie and DJ Rupert, 2014, Presenting efficacy information in direct-to-consumer prescription drug advertisements, Patient Educ Couns, 95(2):271-280.
Proposed Rule
[1] Current federal regulations require that information about the risks of a prescription drug be included in at least the audio portion of a prescription drug advertisement. Many ads also contain text about risks of a drug (information that is provided in both the audio and text is known as dual-modality presentation).
[2] "You are encouraged to report negative side effects of prescription drugs to the FDA. Visit MedWatch or call 1-800-FDA-1088."