Human organ chips for radiation countermeasure development
Scientists are developing models of radiation damage in lung, gut, & bone marrow organs-on-chips and using these models to test MCMs to treat such damage
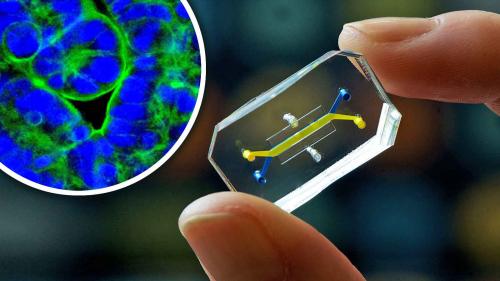
Study expanded to add development and evaluation of new organs-on-chips to aid development of countermeasures for COVID-19
Background | Project description | 2021 update – COVID-19 response | Project outcomes | Publications | Related links
Performer: Wyss Institute for Biologically Inspired Engineering at Harvard University
Principal investigator: Donald E. Ingber, MD, PhD
Initial contract value: $3,588,882
Contract modification value: $1,999,992 (September 2021)
Project dates: September 2019 – March 2023
Background
Acute radiation syndrome (ARS) is an illness affecting a combination of organs that occurs when the body receives a high dose of radiation over a short period of time, as could occur after a nuclear or radiological incident. The U.S. government has identified a need for safe and effective radiation medical countermeasures (MCMs) for military and civilian applications, as well as to mitigate toxicities associated with medical radiotherapies.
The development of MCMs to treat ARS presents complex scientific challenges. For example, ARS may involve many organ systems, which makes it difficult to study candidate MCMs that target the radiation effects on one specific organ system in animal models. Thus, there is a crucial need for more predictive in vitro models of the effects of ionizing radiation on human tissue and organ function. Closing such knowledge gaps to further MCM development and availability is a key goal of the MCMi Regulatory Science Program.
Project description
The Ingber Laboratory at the Wyss Institute has developed human organs-on-chips—microfluidic culture devices that replicate human organ-level pathophysiology. This project builds on past FDA-funded work at the Wyss Institute that led to the development of human models of ARS in bone marrow, intestine, and lung organs-on-chips and the application of these tools to the evaluation of ARS MCMs. Under the current project, the Wyss team will advance the use of human organs-on-chips for MCM development by demonstrating biomimicry and defining critical qualification criteria, helping to reduce the reliance on testing in small animals and nonhuman primates (NHPs).
2021 update – COVID-19 response
In response to the COVID-19 pandemic, FDA partnered with the National Institute of Allergy and Infectious Diseases (NIAID) to develop organs-on-chips and microphysiological systems (MPS) models, to aid development and evaluation of MCMs for COVID-19.
Under a 2021 contract modification, the Wyss Institute, in collaboration with the UK Health Security Agency (formerly Public Health England), will use their organs-on-chips technology to further develop in vitro (human) models of viral infection, propagation, and pathogenicity. Specifically, the project will also include development of NHP organs on chips to provide a bridge from these models to both clinical and nonclinical data, and to better understand comparability to advance MCM assessment.
Project outcomes
Deliverables from this project include:
- Assessing the effects of MCMs (FDA-approved and in development) on reducing radiation damage in male versus female bone marrow chips to determine differences in radiation response by sex
- Analyzing the interplay between human bone marrow organs-on-chips and intestine organs-on-chips that include a complex human gut microbiome in response to radiation exposure
- Recapitulating radiation-induced pneumonitis and response to existing ARS MCMs in the human lung alveolus chip
- Using the Wyss computational discovery pipeline with transcriptomics data to support repurposing existing drugs as radiation MCMs
- Developing NHP (male and female) lung organs-on-chips/MPS
- Developing NHP COVID-19 lung chip models and comparing to human COVID-19 lung chip models
- Exploring and assessing the ability of MCMs (including antiviral therapies) to inhibit viral infection and influence host responses in both NHP and human lung chips.
This project was funded through the MCMi Regulatory Science Extramural Research program and is supported through partnership with the Office of Biodefense Research Resources, and Translational Research, Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH).
Publications
- Human colon-on-a-chip enables continuous in vitro analysis of colon mucus layer accumulation and physiology. Sontheimer-Phelps A, Chou D, Tovaglieri A, Ferrante T, Duckworth T, Fade C, Frismantas V, Sutherland A, Jalili-Firoozinezhad S, Kasendra M, Stas E, Weaver, J, Richmond C, Levy O, Prantil-Baun R, Breault D, Ingber D. Cell Mol Gastroenterol Hepatol. 2019 Nov 25. pii: S2352-345X(19)30163-8. doi: https://doi.org/10.1016/j.jcmgh.2019.11.008
- Human bone marrow disorders recapitulated in vitro using organ chip technology. Chou D, Frismantas, V, Other Wyss, Other Az, SDS authors, Ewart L, and Ingber DE. bioRxiv 458935; doi: https://doi.org/10.1101/458935
- A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Jalili-Firoozinezhad S, Gazzaniga FS, Calamari EL, Camacho DM, Fadel CW, Nestor B, Cronce MJ, Tovaglieri A, Levy O, Gregory KE, Breault DT, Cabral JMS, Kasper DL, Novak R, and Ingber DE. Nature Biomed. Engin. 2019; 3:520-531. doi: https://doi.org/10.1038/s41551-019-0397-0
- Development of primary human small intestine-on-a-chip using patient-derived organoids. Kasendra M, Tovaglieri A, Sontheimer-Phelps A, Jalili-Firoozinezhad S, Bein A, Chalkiadaki A, Scholl W, Zheng C, Rickner H, Dinis ALM, Richmond CA, Li H, Cartwright M, Super M, Breault DR and Ingber DE. Sci. Rep. 2018; 8:2871 doi: https://doi.org/10.1038/s41598-018-21201-7
- Primary human lung alveolus on a chip model of intravascular thrombosis for assessment of therapeutics. Jain A, Barrile, R, van der Meer A, Mammoto A, Mammoto T, DeCeunyck K, Aisku O, Otieno MA, Louden CS, Hamilton G, Flaumenhaft R, Ingber DE. Clin. Pharmacol. Ther. 2018 Feb;103(2):332-340. doi: https://doi.org/10.1002/cpt.742
- Modeling radiation injury-induced cell death and countermeasure drug responses in a human Gut-on-a-Chip. Jalili-Firoozinezhad S, Prantil-Baun R, Jiang A, Potla R, Mammoto T, Weaver JC, Ferrante TC, Kim HJ, Cabral JMS, Levy O, Ingber DE. Cell Death Dis. 2018;9(2):223. doi: https://doi.org/10.1038/s41419-018-0304-8
- Modeling Hematopoiesis and Responses to Radiation Countermeasures in a Bone Marrow-on-a-Chip. Torisawa YS, Mammoto T, Jiang E, Jiang A, Mammoto A, Watters AL, Bahinski A, Ingber DE. Tissue Eng Part C Methods. 2016;22(5):509-15. doi: https://doi.org/10.1089/ten.TEC.2015.0507
- Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Torisawa YS, Spina CS, Mammoto T, Mammoto A, Weaver JC, Tat T, Collins JJ, Ingber DE. Nat Methods. 2014;11(6):663-9. doi: https://doi.org/10.1038/nmeth.2938
- Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Herland A, Maoz BM, Das D, Somayaji MR, Prantil-Baun R, Novak R, Cronce M, Huffstater T, Jeanty SSF, Ingram M, Chalkiadaki A, Benson Chou D, Marquez S, Delahanty A, Jalili-Firoozinezhad S, Milton Y, Sontheimer-Phelps A, Swenor B, Levy O, Parker KK, Przekwas A, Ingber DE. Nat Biomed Eng. 2020 Jan 27. doi: 10.1038/s41551-019-0498-9.
Related links
- Human Organs-on-Chips (Wyss Institute)
- Advancing Alternative Methods at FDA, including FDA definitions of MPS and organs-on-chips
- Human Body-on-Chip platform enables in vitro prediction of drug behaviors in humans (Wyss Institute, January 27, 2020)
- Investigating the human intestinal mucus barrier up-close and personal (Wyss Institute, December 3, 2019)
- Organs-On-Chips Technology Infographic (PDF, 389 KB) - Information about organs-on-chips research from FDA's Center for Food Safety and Applied Nutrition (CFSAN)
- CFSAN’s Work on Organ-Chip Technology
