2011 Biennial Report to Congress on the Food Emergency Response Network (FERN)
September 2011
Report to Congress
Biennial Report to Congress on the Food Emergency Response Network
Submitted pursuant to Section 202 (b) of the FDA Food Safety Modernization Act (P. L. 111-353)
U.S. Department of Health and Human Services
Food and Drug Administration
Executive Summary
This report is intended to satisfy the Food and Drug Administration’s (FDA’s) reporting obligation in Section 202(b) of the FDA Food Safety Modernization Act (FSMA) concerning the Food Emergency Response Network (FERN).
FERN is an integrated, secure laboratory system for Federal, State, and local government agencies engaged in food safety and defense activities. Consisting of 172 Federal, State and local laboratories, FERN is organized to ensure Federal and State inter-agency participation and cooperation in the development and operation of the network. The system is jointly operated by the Department of Health and Human Service’s Food and Drug Administration (HHS/FDA) and the U.S. Department of Agriculture’s Food Safety and Inspection Service (USDA/FSIS), which coordinate with numerous partners, including the Rapid Response Teams (managed through FDA’s Office of Regulatory Affairs), Microbiological and Pesticide Data Programs (managed through USDA’s Agricultural Marketing Service), and the Center for Disease Control and Prevention’s (CDC) Laboratory Response Network.
FERN plays a critical role in food safety and defense by integrating these food-testing laboratories into a network that is able to detect, identify, respond to, and aid in the recovery from emergencies involving biological, chemical, or radiological contamination of food. FERN’s strength lies in allowing participating government agencies to compare, share and coordinate laboratory analysis findings and in strengthening the capacity of State laboratories. FERN cooperative agreement grants supply critical funding to select State member laboratories, increasing national capability and capacity. This support facilitates the ability of these laboratories to serve as first responders during food emergencies.
FERN conducts numerous activities to ensure an integrated and secure laboratory system. It coordinates laboratory methods to promote consistency nationwide, provides training to laboratory professionals on analytical methods, and conducts surveillance testing for specific events or situations. FERN also maintains eLexnet and the FERN Website. eLEXNET acts as the analytical data and official document repository for FERN, while the FERN Website contains a public site and database of laboratory capability and capacity data.
FERN has proven its ability to respond to large scale food emergencies. It has been vital in responding to major outbreaks of foodborne disease attributed to many products, including spinach, pet food, and peanut butter. It has also been vital in aiding in the recovery from emergencies, such as the deep water horizon oil spill and the Japanese nuclear reactor failure, thus helping affected economies and increasing consumer confidence in the food supply.
The FSMA contains many laboratory-related provisions, and FERN will play a vital role in enabling FDA to achieve its mandate. Specifically, FERN will be instrumental in building domestic capacity (Sec. 110), continuing to be a major contributor to the Integrated Consortium of Laboratory Networks (ICLN) (Sec. 203), enhancing foodborne illness surveillance (Sec. 205), and improving the training of State, local, territorial, and tribal food safety officials (Sec. 209).
Overall, the FERN has grown exponentially since its inception, particularly in the ability of member laboratories to participate in Federal surveillance assignments. Beginning with the FDA’s and FSIS’s response to the melamine contamination event in 2007, FERN cooperative agreement State laboratories have participated in Federal assignments (both emergency response and surveillance). This analytical participation by FERN State laboratories has facilitated achieving acceptance of State data by FDA and FSIS for regulatory action.
Another example of the FERN’s success is the recent progress in the implementation of the ICLN, which is lead by the Department of Homeland Security (DHS), with FERN as an integral member. Successful implementation milestones for the ICLN include: development of the Integrated Response Architecture – defining policies for successful operation of ICLN members in the event of an emergency; development of inventories of proficiency testing programs and training programs; and multiple readiness exercises.
Introduction
On January 4, 2011, the President signed into law the FDA Food Safety Modernization Act (FSMA) (Public Law 111-353). Section 202 (b) of the FSMA requires the Secretary of HHS, in coordination with the Secretary of USDA, the Secretary of DHS, and State, local, and tribal governments, not later than 180 days after the date of enactment of the FSMA and biennially thereafter, to prepare a report that describes “progress in implementing a national food emergency laboratory network that—
- provides ongoing surveillance, rapid detection, and surge capacity for large-scale food-related emergencies, including intentional adulteration of the food supply;
- coordinates the food laboratory capacities of State, local, and tribal food laboratories, including the adoption of novel surveillance and identification technologies and the sharing of data between Federal agencies and State laboratories to develop national situational awareness;
- provides accessible, timely, accurate, and consistent food laboratory services throughout the United States;
- develops and implements a methods repository for use by Federal, State and local officials;
- responds to food-related emergencies; and
- is integrated with relevant laboratory networks administered by other Federal agencies.”
The Secretary is to submit the report to the relevant committees of Congress and to post it on the HHS website.
The following report is the first biennial report in response to this mandate since the signing of the FSMA on January 4, 2011.
Background
Following September 11, 2001, attention has focused on the risk of bioterrorism threats, particularly with regard to the nation's food supply. The Public Health Security and Bioterrorism Preparedness and ResponseAct of 2002 directed HHS/FDA responsibility for a wide-ranging program to protect the American public from attacks on the food supply. FERN was developed in 2004 in response to Homeland Security Presidential Directive 9 (HSPD-9), which established food as a critical infrastructure for the United States and charged agencies with developing a national food testing network.
FERN integrates the nation's food-testing laboratories at the Federal, State and local levels into a network that is able to detect, identify, respond to and recover from emergencies involving biological, chemical, or radiological contamination of food. The FERN structure ensures Federal and State inter-agency participation and cooperation in the formation, development, and operation of the network.
FERN works to enhance food safety by:
- Allowing rapid detection of threat agents in the American food supply;
- Preparing the nation's laboratories to be able to respond to food-related emergencies;
- Offering significant surge capacity that enables laboratories to be activated as needed during emergencies, thus strengthening the nation's response towards widespread complex intentional or inadvertent food contamination; and
- Enhancing the ability of the country to restore confidence in the food supply following a threat or an actual emergency targeting the nation's food supply.
FERN Structure
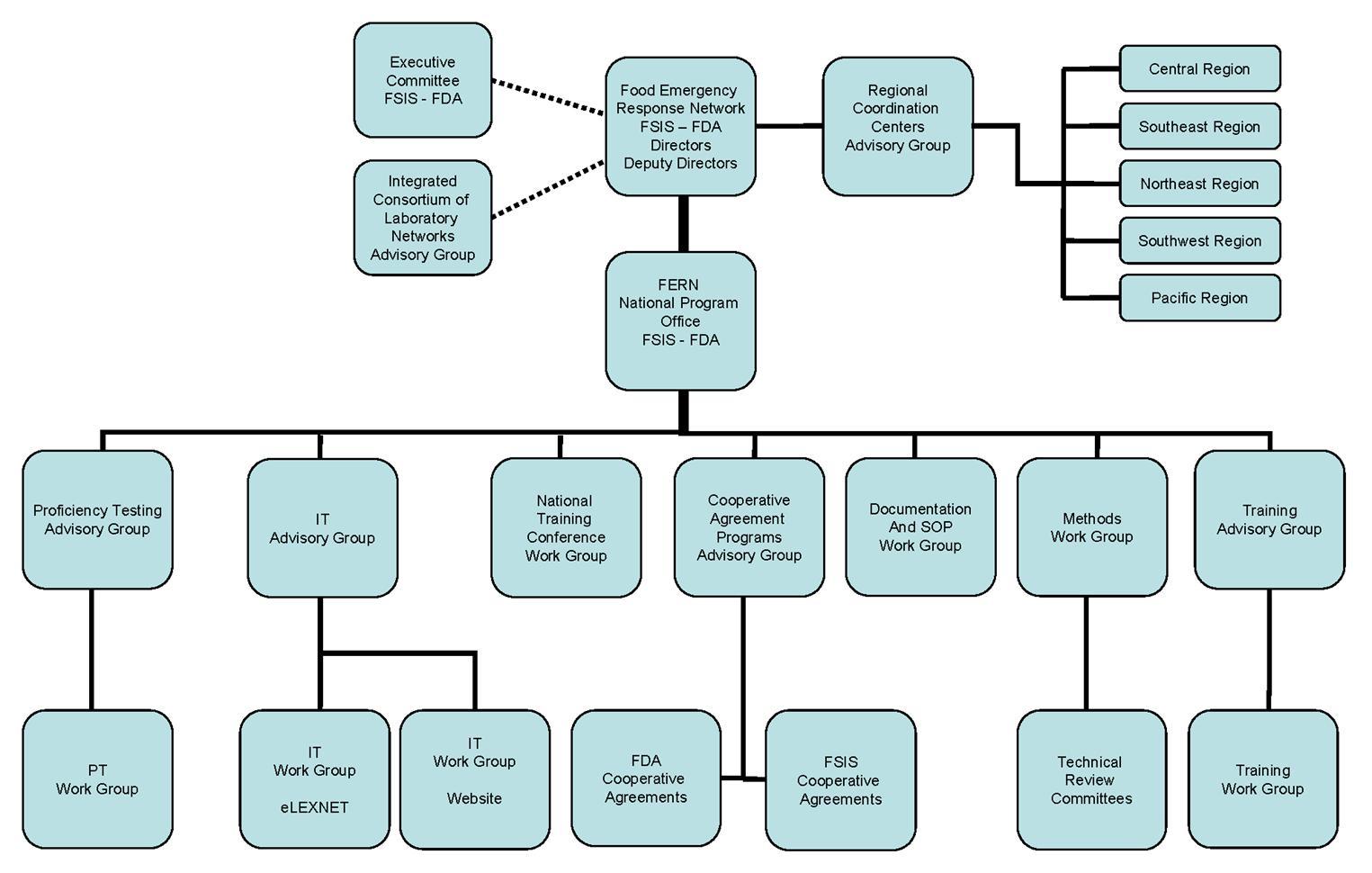
FERN consists of five components: the Executive Committee/FERN Directors; the National Program Office (NPO); the Support Programs; the Regional Coordination Centers (RCC’s); and the FERN laboratories comprising all participating Federal, State, and local laboratories.
Executive Committee/FERN Directors
The FERN Executive Committee is co-chaired by senior executives from the USDA/FSIS and HHS/FDA and the day-to-day operations of FERN are a joint venture between the two Agencies. The Executive Committee provides senior input and leadership from each Agency to the FERN Directors and NPO. The FERN Directors, one from FDA and one from FSIS, work closely with the Executive Committee and direct the National Program offices. The Directors are also members of the Network Coordinating Group (NCG) of the ICLN.
National Program Office
The NPO has two locations: the FDA office is in Rockville, MD, and the FSIS office is in Athens, GA. Both are responsible for the day-to-day operation of FERN, which includes managing the FERN support programs, coordinating national laboratory emergency responses, and providing oversight of the RCC.
Support Programs
FERN has five support programs: (1) methods coordination, (2) training, (3) proficiency testing, (4) surveillance assignments, and (5) electronic communications and collaborations. Each of the programs supports the three analytical disciplines: chemical, microbiological, and radiological.
- The FERN Methods Coordination Committee promotes consistency and quality of laboratory methods. It is responsible for determining method priorities for FERN, generating guidelines for submitting methods and soliciting method submissions from member laboratories, and reviewing and approving submissions to be considered as FERN methods. FERN also participates in method harmonization workgroups with other laboratory networks and programs, such as the CDC’s Laboratory Response Network (LRN), through the Integrated Consortium of Laboratory Networks.
- The FERN training program promotes a well-trained cadre of laboratory professionals. It offers courses through both the FDA training program and six FSIS training centers, which are co-located with member laboratories and funded through cooperative agreements. A wide variety of training courses are offered in chemical, microbiological, and radiological methods. In the last six years, FERN has developed more than 80 training courses, and more than 1,000 laboratory staff from Federal, State, local, and international food laboratories have participated in training exercises over the years.
- The FERN proficiency testing program serves a quality control function. It offers microbiology, chemistry, and radiochemical proficiency tests. The proficiency tests alternate between food safety and food defense threat agents, and also rotate the choice of food matrices based on current events and risk assessments. The FERN proficiency testing program is active with other networks, participating in and providing harmonized testing for the Department of Homeland Security’s Integrated Consortium of Laboratory Networks (ICLN). Participating networks have included the LRN-biological, LRN-chemical, and the Environmental Protection Agency’s lab network.
- FERN participates in surveillance assignments for specific events or situations, such as during the Republican and Democratic National Conventions of 2008 and the Presidential inauguration in 2009. FERN also assists in the ongoing targeted surveillance of the National School Lunch Program and imported food commodities.
- Lastly, FERN coordinates electronic communications and collaborations through two separate systems: eLEXNET and the FERN Website. eLEXNET acts as the analytical data and official document repository for FERN. The FERN Website contains a public site and database of laboratory capability and capacity data, called LabDIR. It also contains registration modules for trainings, meetings and proficiency tests.
Regional Coordination Centers
The FERN RCCs are located in each of the five FDA regions across the United States and are staffed by both FDA and FSIS personnel. The primary responsibility of the RCCs is to identify needs of the region and convey those needs to the NPO. RCCs establish operational and communication guidelines within each FERN region; communicate their objectives, policies and current activities; enhance collaboration among FERN laboratories within a region; and provide an opportunity for individual regions to tailor response plans to their State policies and regional needs.
FERN Laboratories
FERN laboratories are able to respond to emergencies involving biological, chemical and radiological contamination of food. Since 2004, the FERN has successfully worked to strengthen the ability of our nation’s food testing laboratories to respond to a food contamination event through several initiatives, including increasing the capability and capacity of member laboratories by using FERN Support Programs, as well as encouraging new laboratories to join the network.
FERN membership is open to public (Federal, State, local, and tribal) food testing laboratories that perform regulatory and/or diagnostic analytical work. As of April 2011, FERN has 172 laboratory members (39 Federal, 116 State, and 17 local), located in all 50 States and Puerto Rico. FERN member laboratories represent the large majority of food testing laboratories in the U.S., including public health, agriculture, veterinary diagnostic and environmental laboratories. At this point, it is estimated that the FERN membership represents about 85% of all eligible food regulatory laboratories in the U.S.
The growth of FERN since its inception in 2004 is clearly documented in the table below:
|
Year
|
Number of FERN Member Laboratories
|
|---|---|
|
2004
|
8
|
|
2005
|
88
|
|
2006
|
93
|
|
2007
|
134
|
|
2008
|
150
|
|
2009
|
158
|
|
2010
|
165
|
|
2011
|
172
|
Membership in the FERN is voluntary, and FERN requires that all prospective members submit a Laboratory Qualification Checklist, containing basic laboratory capacity and capability information, and be willing to participate in FERN activities and directives. This checklist is reviewed by the FERN NPO to determine if a laboratory meets the criteria for participation in FERN. Upon acceptance into the FERN, laboratories are then expected to complete a detailed assessment, with a near 100 per cent compliance, enabling the NPO to generate rapid laboratory capacity and capability assessment (housed electronically through the FERN Website, in LabDIR), which must then be updated and verified on an annual basis to maintain active FERN membership status. This laboratory assessment inventory tool is used to quickly assess FERN capabilities and capacities for reacting to food emergencies. The FERN NPO queries the database for a specific analytical need and determines the laboratories able to assist, as well as the laboratories’ self-assessed sample capacity. FERN member laboratories complete network-wide capacity and capability inventories.
FERN laboratories are encouraged, although not required, to participate in any and all FERN activities. An exception to this rule applies to laboratories with FERN Cooperative Agreements, which are required to participate in a number of specified FERN Support Programs and Activities.
FERN Cooperative Agreements
FERN Cooperative Agreements increase national capability and capacity by awarding funds to State member laboratories. Funds usually support procurement of equipment and dedicated personnel, as well as projects covered under the cooperative agreement. These cooperative agreements work to address harmonization of analytical platforms, methods and other laboratory operations that support laboratory and data confidence and that can then be applied on a larger scale across FERN. The dedicated personnel serve both the FERN programs and the State programs.
These cooperative agreement laboratories are a critical component of any large-scale food emergency response. The chemistry laboratories have been integral participants in such outbreaks and events as the melamine contaminations and the Deepwater Horizon oil spill. The microbiological laboratories have participated in contamination events such as E. coli in spinach (2006), Salmonella in peppers (2008), and Salmonella in peanut butter (2009). The radiological laboratories are currently involved in testing foods related to the Japanese nuclear reactor failure. All of the analytical work done in response to these events serves to promote the use of food regulatory data across all Agencies, Federal, State, and local, by utilizing the same laboratory standards as mandated in the FSMA under Section 203(a)(1).
Cooperative Agreement Requests for Applications (RFAs) are published in the Federal Register. FERN cooperative agreements are awarded and managed separately through both FDA and FSIS. FSIS awards and manages 25 cooperative agreements, funding both microbiological/chemical projects and program support activities. FDA awards and manages a total of 33 cooperative agreements: 15 microbiological, 14 chemical and 5 radiological (see the chart below for a historical accounting of the FDA and FSIS managed FERN Cooperative Agreement Program (CAP)).
|
Fiscal Year
|
FDA FERN Cooperative Agreements
|
FSIS FERN CAP
Agreements
|
|---|---|---|
|
2005
|
8 Chemistry
|
18 Microbiology
|
|
2006
|
8 Chemistry; 2 Radiochemistry
|
17 Microbiology
|
|
2007
|
8 Chemistry; 5 Radiochemistry
|
17 Microbiology
|
|
2008
|
11 Chemistry; 5 Radiochemistry
|
21 Microbiology
|
|
2009
|
14 Chemistry; 5 Radiochemistry
|
25 Microbiology & 4 Program Support
|
|
2010-Present
|
15 Microbiology; 14 Chemistry;
5 Radiochemistry
|
25 Microbiology/Chemistry & 4 Program Support
|
FERN Federal Partners
Throughout the evolution of FERN, the majority of the 172 member laboratories have been simultaneously fulfilling the requirements of several Federal programs with respect to food safety. Recognizing this, FERN has been pursuing opportunities for intra- and inter-agency coordination and leveraging. The benefits of doing so are three-fold: to maximize efficiencies and effectiveness of Federal resources; to streamline processes within our State laboratories by communicating similar messages and harmonizing priorities; and to improve relations between Federal and State partners by prioritizing concerns.
FERN routinely consults with Federal partners for expertise and input regarding FERN Support Programs and activities. The FERN NPO coordinates a monthly conference call with the FDA Center for Food Safety and Applied Nutrition (CFSAN) for transparency and cross-communication purposes. The FERN NPO also routinely communicates with other laboratory networks and programs to increase transparency and communication, as well as to identify potential areas for harmonization of activities and leveraging. Such partners include: Rapid Response Teams (managed through FDA’s Office of Regulatory Affairs), Microbiological and Pesticide Data Programs (managed through USDA’s Agricultural Marketing Service) and the CDC Laboratory Response Network (LRN).
FERN has an inter-agency agreement with the Department of Defense to supply reagents from its Critical Reagent Program for FERN analytical platforms and is working on an interagency agreement with USDA’s Agricultural Marketing Service (AMS) to develop a program to harmonize methodologies so that samples taken by FERN member State laboratories for AMS surveillance testing of commodities for pathogens and pesticides could also satisfy the requirements for FDA surveillance assignments.
The FERN NPO is also an active member of the Department of Homeland Security’s ICLN, and several NPO staff are driving forces on ICLN Subgroups, such as Proficiency Testing/Quality Assurance, Methods, and Information Technology (IT). The FERN Directors participate in the ICLN Network Coordination Group to promote transparency and communication about FERN activities, and discuss how FERN can better work with other ICLN members to meet the harmonization and standardization criteria, as set by the ICLN. FERN is an active participant in joint proficiency testing and tabletop exercises.
Recent progress in the implementation of the ICLN, with FERN as an integral member, includes: development of the Integrated Response Architecture consisting of defining policies for interoperability; inventories of networks’ proficiency testing programs and training programs; and readiness exercises. Incident response matrices have been developed, identifying roles and gaps. Data exchange discussions are underway, seeking to identify data fields and IT gaps.
Progress in Implementing a National Food Emergency Network
The following section describes FERN’s progress in implementing a national food emergency laboratory network that addresses the six areas enumerated in sections 202(b)(1)-(6) of FSMA:
(1) provides ongoing surveillance, rapid detection, and surge capacity for large-scale food-related emergencies, including intentional adulteration of the food supply;
Large-scale food emergencies are complex and usually require a multi-tiered response that includes rapid detection and the ability to test many samples. Initial rapid detection of the pathogen or contaminant may require new or modified methodologies that must be developed, improved upon, and ultimately verified for use in the laboratories. The FERN is able to call upon the nation’s leading food testing experts, both Federal and State, to assist in this method development, and the subsequent necessary roll-out of the technology to the testing laboratories. One example of this work is the rapid method developed and used for detecting E coli O157:H7 in spinach (2006). This method was developed by FERN and FDA, quickly validated for use, approved by the FERN Methods Committee, and implemented in the laboratories for use during the outbreak. This new rapid method cut several days off of the existing method, and was supported with reagents and supplies from the FERN Storeroom.
FERN’s ability to provide ongoing surveillance, rapid detection, and surge capacity was demonstrated during the two melamine-related outbreaks of 2007 and 2008-09. In 2007, the companion animal illnesses and deaths were linked to melamine-contaminated pet foods with ingredients imported from China, and in 2008 melamine resurfaced as an adulterant added to infant formula and other food products, e.g., chocolate, -- again linked to products in China. During the 2007 outbreak, FERN laboratories were activated to assist with sample analyses (surge capacity). FERN chemistry laboratories, working with the FDA Forensic Chemistry Center, the FSIS Western Lab and the National Animal Health Network (NAHLN) developed a screening method for melamine and its analogs. This method was used throughout this melamine event by FDA and FSIS laboratories and the eight FERN Chemistry Cooperative Agreement Program Laboratories that participated in the FDA Protein Surveillance Assignment. In 2008-09, FERN laboratories were once again activated to assist with sample analyses, and new, faster methods were developed and validated by FDA for infant formula and other dairy products. These new methods were more sensitive than the previous melamine method and could therefore detect smaller amounts of the chemical. FERN laboratories analyzed a total 340 samples (about 20% of all assignment collections), and found 14 samples contaminated with melamine and/or its analogs. The use of the FERN Chemistry cooperative agreement laboratories was a key factor in clearing an FDA sample backlog, which arose due to very high collection rates.
(2) coordinates the food laboratory capacities of State, local, and tribal food laboratories, including the adoption of novel surveillance and identification technologies and the sharing of data between Federal agencies and State laboratories to develop national situational awareness; FERN coordinates the food laboratory capacities of State, local, and tribal food laboratories through the five RCCs situated across the U.S. These regional coordinators interact with the area laboratories, assessing capacity and capability for the region, and reporting this information back to the NPO. They also include assessments of laboratories’ training needs, potential method issues, and any needs the laboratories might have bearing on readiness. The regional coordinators work closely with the Regional Coordination Oversight group to ensure continuity of effort between the regions, and provide communication with the National Program Office. RCC’s also disseminate information from the NPO, including situational awareness of national events and FERN activations, new method roll-outs, reagent issues, training opportunities, and general FERN business.
Communication is a critical component of a network, and FERN works diligently to provide the information necessary. Many different formats and venues are utilized to ensure that communication is effective and flows to and from the National Program Office. These include:
- A FERN Newsletter published every two months.
- FERN-wide conference calls used for routine communication as well as for events and activations.
- Regional calls – monthly and ad hoc.
- Monthly calls with laboratories participating in Cooperative Agreement.
- State participation on working groups and oversight groups.
- FERN National Training Conferences that include general sessions and extensive breakout sessions. These meetings are very well attended, and may be the largest meeting of public food regulatory laboratories held yearly.
(3) provides accessible, timely, accurate, and consistent food laboratory services throughout the United States;
FERN works diligently through the FERN Support Programs to provide rapid, accurate, and consistent analytical testing. The NPO works with the member laboratories to promote the use of validated rapid methods, including the development and validation of new methods for network usage. The NPO Proficiency Testing program provides proficiency and competency testing to all of its member laboratories. To date, more than 30 proficiency tests have been provided, with more than 1,500 participants. Additionally, FERN surveillance assignments, usually about four per year, provide all participants the opportunity to engage in the testing of actual samples that the FERN NPO can review and assess. The diligence paid to communication by the FERN also works to promote timely, accurate, and consistent laboratory services throughout FERN.
One example of FERN’s ability to reduce the time needed for sampling is, as cited above, the rapid method developed and used for detecting E coli O157:H7 in spinach in 2006. This new rapid method cut several days off of the time required to obtain sample results.
(4) develops and implements a methods repository for use by Federal, State and local officials;
The FERN develops and implements a methods repository for use by Federal, State, and local officials through the Methods Coordination Committee, which is responsible for generating Method Validation and Submission guidelines, determining method priorities for the FERN, soliciting method submissions from member labs, and reviewing and approving submissions as FERN methods. FERN also participates in method harmonization workgroups with other laboratory networks and programs, such as the CDC Laboratory Response Network, through the Integrated Consortium of Laboratory Networks.
(5) responds to food-related emergencies;
FERN has been activated for several microbiological and chemical emergencies, providing surge capacity for both Federal and State lead investigation and response efforts. In responding to a food-related emergency, FERN can activate any of a variety of functions, from providing technical guidance and reagents to directing sample collection and analysis by member laboratories. Events for which FERN components have been activated include the following emergencies, which are detailed below:
- E.coli in Spinach Outbreak (2006)
- Melamine in Pet Food (2007)
- Salmonella Saintpaul in Peppers (2008)
- Melamine in Infant Formula and Milk-Based Products (2008-2009)
- Salmonella Typhimurium in Peanut Butter (2009)
- Deepwater Horizon Oil Spill (2010)
- Japan Nuclear Reactor Response (2011)
E. coli O157:H7 in Spinach Outbreak (2006)
In August 2006, public health laboratories in the United States identified 199 cases of
E. coli O157:H7 in 26 States linked to the consumption of fresh spinach. Overall, 51% of the cases were hospitalized and 16% had kidney failure. The FERN was activated during this outbreak and provided access to reagents, technical guidance, screening and culture methods. FERN monitored the situation to determine the need for surge capacity, and a harmonized FERN/LRN/CDC method was quickly reviewed and published electronically on the eLEXNET system for use in FERN laboratories. This process was expedited, reducing the method approval process from weeks to a few days.
Melamine in Pet Food (2007)
In the spring of 2007, there were reports of companion animal illness and death linked to pet foods with ingredients imported from China and melamine. Melamine and cyanuric acid were identified as the hazardous adulterants. The contamination issue spread when melamine was found to be present in other animal feed products. FERN was activated, and eight FERN Chemistry Cooperative Agreement Program Laboratories participated in the FDA Protein Surveillance Assignment. With Federal and State assistance, the FDA Forensic Chemistry Center, the FSIS Western Lab, and National Animal Health Laboratory Network (NAHLN) developed a new screening method for melamine and its analogs that was used in this surveillance assignment. There was also a collaborative effort among States, USDA, and FDA to develop more sensitivemethods for the analysis of melamine and cyanuric acid in animal tissue (fish, pork, etc.).
FERN Laboratories that participated in FERN-directed analyses included:
- FDA Forensic Chemistry Center
- Arizona Department of Health Services
- California Animal Health and Food Safety
- Connecticut Agricultural Experiment Station
- Florida Department of Agriculture and Consumer Services
- State Hygienic Laboratory at the University of Iowa
- Minnesota Department of Agriculture
- New Hampshire Public Health Laboratory
- Virginia Division of Consolidated Laboratory Services.
Salmonella Saintpaul in Peppers (2008)
During the spring and summer of 2008, the second largest outbreak of Salmonella Saintpaul ever reported in the United States was identified by PulseNet (>1400 cases) in 43 States, DC, and Canada. Initial epidemiologic reports indicated tomatoes as a potential common food source, but further investigation by the FERN and other public health agencies and laboratories led to the identification of an indistinguishable Saintpaul isolate from a jalapeño pepper, which was traced back through a distribution center in Texas to a grower in Mexico. FERN was activated, and 12 FERN microbiology laboratories analyzed pepper, cilantro and basil samples collected through State and FDA coordinated efforts. FERN testing augmented and complemented concurrent testing by FDA’s Office of Regulatory Affairs. In all, FDA tested a total of 1,618 samples, and found 75 positives (39 unique Salmonella serotypes), and FERN tested a total of 290 samples and found 13 positives (six unique Salmonella serotypes).
FERN Laboratories that participated in FERN-directed analyses included:
- Florida Department of Agriculture and Consumer Services
- Pennsylvania Department of Agriculture
- Wisconsin Department of Agriculture, Trade and Consumer Protection
- Michigan Department of Agriculture
- New York Department of Agriculture and Markets
- Indiana State Department of Health
- California Department of Public Health Microbial Disease Lab
- California Department of Public Health Food and Drug Lab
- Minnesota Department of Agriculture
- Texas Department of State Health Services
- North Carolina Department of Agriculture
- Massachusetts State Laboratory Institute.
Melamine in Infant Formula and Milk-Based Products (2008-2009)
In 2008, melamine resurfaced as an adulterant added to infant formula and other food products, e.g., chocolate, linked to producers in China, and the FERN was activated (October, 2008). New testing methods were developed and validated by FDA for infant formula and other dairy products. These methods were more sensitive than the melamine methods used in 2007 for pet food sampling. These methods were used by USDA, FDA, FERN, and the States in the analysis of milk-based products for melamine.
Since 2007, USDA and FDA have analyzed thousands of samples for melamine and its analogs, and FERN chemistry laboratories assisted FDA in the CFSAN Melamine Import Assignment for the analysis of milk and soy protein based samples (issued 11/24/08). FERN laboratories analyzed a total 340 samples (about 20% of all assignment collections), detected 14 instances of melamine and/or analogs, and were a key factor in clearing an FDA sample backlog, which arose due to very high collection rates.
FERN Laboratories that participated in FERN-directed analyses included:
- FDA Forensic Chemistry Center
- Arizona Department of Health Services
- California Animal Health and Food Safety
- Connecticut Agricultural Experiment Station
- Florida Department of Agriculture and Consumer Services
- Hawaii Department of Agriculture
- Hawaii Department of Health
- Illinois Department of Agriculture
- Maryland Department of Agriculture
- Minnesota Department of Agriculture
- Montana Department of Agriculture
- New Hampshire Public Health Laboratory
- New Jersey Department of Health and Senior Services
- University of Iowa Hygienic Laboratory
- Virginia Division of Consolidated Laboratory Services
- Washington State Public Health Laboratory
- West Virginia Department of Agriculture.
Salmonella Typhimurium in Peanut Butter (2009)
An outbreak in the fall of 2008 and winter 2009 led to 714 cases of Salmonella typhimurium infection in 46 States linked to consumption of products containing peanut butter produced by the Peanut Corporation of America (PCA) plant in Blakely, GA. More than 2,800 products were identified that contained peanut butter produced by this plant. FERN laboratories were not officially activated, but there were strong contributions to the investigation from FERN laboratories in MN, MI, OH, and CT. FERN Storeroom reagents were made available as well as technical guidance, methods, and molecular fingerprinting (PFGE) support. Additionally, FERN assisted the North Carolina Department of Agriculture (a FERN member laboratory) in coordinating a sample overflow in their laboratory.
Deepwater Horizon Oil Spill (2010)
The Deepwater Horizon Oil Spill, caused by an explosion on a drilling rig on April 20, 2010, released several million barrels of crude oil into the Gulf of Mexico until the wellhead was capped on July 15, 2010. The oil from the Deepwater Horizon well contaminated a large number of Gulf State fisheries in Louisiana, Alabama, Mississippi, and Florida, which resulted in an almost total shutdown of the industry. National Oceanic and Atmospheric Administration (NOAA), FDA, and State governments cooperated in an effort to close State and Federal waters to commercial fishing as a result of the public health threat from contamination of seafood by polyaromatic hydrocarbons (PAHs), the principal toxicologic concern. To address this threat, a detailed protocol for reopening State and Federal waters was implemented involving an extensive chemical testing program. This protocol outlined specific levels of concern for each PAH that the labs were tasked with measuring.
FERN laboratories (including FDA Office of Regulatory Affairs Field Laboratories and FERN Cooperative Agreement Laboratories) were used to analyze these samples. Two methods were used in the chemistry testing portion of the protocol -- a method developed by NOAA and a PAH screening method developed by the FDA Forensic Chemistry Center. FERN Cooperative Agreement Program laboratories (Connecticut Agricultural Experiment Station and Minnesota Department of Agriculture) were critical to the development and implementation of this PAH screening procedure. Without the analyses performed by the FERN laboratories and the development of a rapid screening method, the safe and rapid reopening of the Gulf State fisheries would not have been possible.
Because of the extensive need for sampling, FERN was activated in May 2010 to enable all FERN laboratories, not just those that are funded through cooperative agreements, to conduct sampling. FERN began to assess network capabilities and capacities for the NOAA method, and selected FERN Cooperative Agreement Program laboratories, which were tasked with analyzing samples using this methodology. Additionally, the FERN Storeroom ordered and stocked standards and reagents required for performing the NOAA method. Reagent requests were filled on a prioritized basis, with first priority going to Gulf State laboratories and laboratories conducting FERN directed testing. Over the course of the FERN Activation, 307 finfish, crab, oyster and shrimp samples from Florida, Alabama, Mississippi and Louisiana were analyzed for PAHs using the liquid chromatography-fluorescence detection (LC-FLD) alternative screening method as part of the State reopening process (performed by FDA Forensic Chemistry Center, Connecticut Agricultural Experiment Station, and the Minnesota Department of Agriculture). Of those samples, 66 received parallel NOAA method analysis. In addition to the reopening samples, 88 State baseline samples were analyzed using the NOAA method. The FERN was deactivated for this incident in November, 2010.
FERN Laboratories that participated in FERN-directed analyses included:
- FDA Arkansas Regional Laboratory
- FDA Denver District Laboratory
- FDA Forensic Chemistry Center
- FDA Kansas District Laboratory
- FDA Southeast Regional Laboratory
- Arizona Department of Health Services
- California Animal Health and Food Safety
- Connecticut Agricultural Experiment Station
- Florida Department of Agriculture and Consumer Services
- Minnesota Department of Agriculture
- Wisconsin Department of Agriculture, Trade and Consumer Protection.
Japan Nuclear Reactor Response (2011)
On March 11, 2011, a magnitude 9.0 earthquake struck 130 miles off the eastern coast of Honshu, Japan's largest island. The ensuing tsunami resulted in loss of control of nuclear reactors in the Fukushima Daiichi complex. This culminated in radionuclide leakage to the atmosphere and ocean, causing widespread radioactive contamination of residential areas, agricultural land and coastal waters. As part of the FDA effort to monitor and respond to potential food-borne radiation contamination of imported commodities from Japan, FERN was activated and readied their five Radiation Cooperative Agreement Program (Rad-CAP) laboratories. The FERN labs’ primary role was to assist FDA laboratories in the event that FDA sample testing capacity was exceeded. In preparation for this role, these laboratories worked closely with the FERN National Program Office, scientists at the FDA Winchester Analytical and Engineering Center (WEAC) laboratory and at the FDA Center for Food Safety and Applied Nutrition to validate emergency methodologies for food-borne gamma ray-emitting radionuclide contaminants.
FERN Laboratories that participated in the FERN activation:
- Maryland Department of Health and Mental Hygiene
- New York State Department of Health
- Texas Department of State Health Services Laboratory
- Washington State Public Health Laboratories
- Wisconsin State Laboratory of Hygiene
(6) is integrated with relevant laboratory networks administered by other Federal agencies.
The FERN NPO routinely communicates with other laboratory networks and programs to increase transparency and communication, as well as to identify potential areas for harmonization of activities and leveraging. Such partners include: Rapid Response Teams (managed through FDA Office of Regulatory Affairs), Microbiological and Pesticide Data Programs (managed through USDA Agricultural Marketing Service), and the CDC LRN.
The FERN NPO is also an active member of the Department of Homeland Security’s ICLN, and several NPO staff are driving forces on ICLN Subgroups, such as Proficiency Testing/Quality Assurance, Methods, and Information Technology (IT). The FERN Directors participate in the ICLN Network Coordination Group to promote transparency and communication about FERN activities, and discuss how FERN can better work with other ICLN members to meet the harmonization and standardization criteria, as set by the ICLN. All of these activities clearly depict the FERN’s integration with relevant laboratory networks administered by other Federal agencies.
Recent progress in the implementation of the ICLN, with FERN as an integral member, includes: development of the Integrated Response Architecture – defining policies for interoperability; inventories of networks’ proficiency testing programs and training programs; and readiness exercises. Incident response matrices have been developed, identifying roles and gaps. Data exchange discussions are underway, seeking to identify data fields and IT gaps
Conclusion
FERN integrates the nation's food-testing laboratories at the Federal, State, local, and tribal levels into a network that is able to respond to emergencies involving biological, chemical, or radiological contamination of food. The FERN network focuses on preparedness through awareness, surveillance, prevention, and capacity-building programs and seeks to build response and recovery surge capacity. FERN provides for an early means of detecting threat agents in the U.S. food supply and prepares the nation's laboratories to be able to respond to food-related emergencies. FERN offers significant surge capacity that strengthens the nation's response towards widespread complex intentional or inadvertent food contamination. FERN has shown its ability to respond to the large-scale food emergency events of the last five years. This ability of the FERN network to provide a cohesive response serves to enhance the ability of the Government to restore confidence in the food supply following a threat or an actual emergency targeting the nation's food supply.
