GUIDANCE DOCUMENT
Draft Guidance for Industry: Regulatory Submissions to OFAS, Part III Electronic Format December 2013
Not for implementation. Contains non-binding recommendations.
Contains Nonbinding Recommendations
Draft — Not for Implementation
December 2013
Table of Contents and Introduction (Part I)
Information for all regulatory submissions
- Part I: Introduction
- Part II: Common Elements
- Part III: General Considerations - Electronic Format
- Part IX: FDA References
- Part X: Appendices
- Quick Links to Forms, Instructions and Downloadable Folders
Regulatory submissions for program areas
- Part IV: Food or Color Additive Submissions
- Part V: Food Contact Substance Submissions
- Part VI: GRAS Notices
- Part VII: Biotechnology Final Consultations
- Part VIII: New Protein Consultations
All parts [Printable PDF Version]
III. General Considerations about Regulatory Submissions in Electronic Format
- General Information
- Transmission of a Regulatory Submission in Electronic Format
- File Formats and Names in an Electronic Regulatory Submission
- Roadmaps
- Organizational Considerations for Files Included in Electronic Regulatory Submissions
- Preparation of Amendments, Updates, and Supplements in Electronic Format
- Electronic Signatures
A. General Information
-
Should I use a password to protect a regulatory submission in electronic format?
No. Our internal security and archival processes will maintain the integrity of the files received.
-
Should I check a regulatory submission in electronic format for computer viruses?
Yes. The forms provide a checkbox prompting you to confirm that the submitted files have been checked and are free of computer viruses. If you are sending a cover letter refer to Section II.F above.
-
What is the size limit of electronic files I submit?
The size limit of electronic files you submit is 50 Megabytes (MB). You should divide any files larger than 50 MB into multiple files.
B. Transmission of a Regulatory Submission in Electronic Format
-
What is the FDA Electronic Submission Gateway (FDA ESG)?
The FDA ESG is an agency-wide portal mechanism for receiving submissions in electronic format. The FDA ESG enables the secure submission of regulatory information for review and provides for the receipt of a document.
-
How do I use the FDA ESG?
Complete instructions for use of the FDA ESG are available in FDA’s Web site (Ref. 1). As indicated on the ESG Web site, before you send actual submission you have to first set up a test account and send a test submission. Then you have to apply for, and setup, a production account to send actual submissions through the production system account.
-
Is the FDA ESG the only mechanism available for me to transmit my regulatory submission in electronic format?
No. You also may transmit a regulatory submission in electronic format by providing your electronic files on physical media (e.g., CD-ROM or DVD) and transmitting these physical media by means such as mail or a courier service.
-
What recommendations apply when I use physical media (such as CD-ROMs or DVDs) to transmit my regulatory submission in electronic format?
A regulatory submission transmitted in electronic format on physical media should be accompanied by a paper copy of any form or cover letter that you sign and provide information about the physical media.
You may “zip” files to provide as much information as possible on a single disc (or other physical medium). If you need to use more than one disc (or other physical medium), you should label each item (e.g., Disc 1 of 4, Disc 2 of 4, etc.). Files on each disc should be placed in the appropriate folders of the applicable roadmap. For example, large safety studies may be included in a separate disc; you would place the studies in the appropriate subfolders in the Safety folder of the roadmap; the rest of the roadmap would remain empty on that disc (do not submit duplicates of any files).
C. File Formats and Names in an Electronic Regulatory Submission
- What file formats should I use for my regulatory submission?
The type of file format to use depends on the type of data or information you are submitting. Table III-1 lists the file formats we recommend you use for the most common types of data and information. We generally are able to access formats listed in Table III-1 and data sets that are compatible with XML (extensible markup language) format. We recommend that you contact us before submitting data or information in any file format not listed in Table III-1. Doing so should minimize problems with your electronic submission.
The different Centers in FDA are in varying stages of implementing a pilot program for accessing files in Standard for Exchange of Nonclinical Data (SEND) and Study Data Tabulation Model (SDTM) (Ref. 14). The likelihood that OFAS will be able to receive files in SEND or SDTM increases as time goes on. Therefore, we recommend that you contact us before submitting files in SEND or SDTM formats. We can also receive files containing study data in XML format.
Additional information relevant to the use of electronic collection of clinical trial data is available in the Guidance to Industry - Computerized Systems Used in Clinical Investigations (Ref. 12).
Table III-1
Recommended File FormatsType of Data or Information Recommended File Format General text Portable Document Format (PDF) Primary data or summary data (e.g., toxicological study reports) PDF for tabular data, in addition to PDF, provide in spreadsheet format (e.g., Excel), if available. Chemical structures Molfiles (.mol) (preferred); or ChemDraw files (.cdx); or Structure & data files (.sdf) (Chemical structures should be provided as images within PDF files) Animal Study Data In addition to PDF for full study reports, use templates (Ref. 13) when available and transmit the information in the completed template in PDF; OR Use Standard for Exchange of Nonclinical Data (SEND) (Ref. 14) Clinical Trial Data Generated by Computerized Systems Study Data Tabulation Model (SDTM) (Ref. 14) References PDF - What recommendations apply to files prepared in PDF format?
We recommend that you create PDF files directly from electronic source documents (rather than from scanned images) whenever possible. Such files require less memory space than scanned image files. Such files also help assure access to, and accurate searching of, text, tabular data and graphical objects.
If you scan a document to create a PDF file, we recommend that you capture text by optical character recognition (OCR) software so that the text of the resulting electronic documents is reasonably accessible and searchable.
- How should I name files in a regulatory submission transmitted in electronic format?
You should name a file in a way that will help us to see what is in the file and when the file was prepared or submitted. Doing so will help us to store the file in our database in a way that our reviewers can easily find it and will improve the efficiency of our review.
In general, you should name a file using both generic elements (which relate to the type of information in the file, such as a toxicity study) and specific elements (which relate to your particular submission, such as the name of the substance that is the subject of the submission). The specific recommended conventions for naming files vary depending on factors such as:
- The type of data or information;
- Whether the data or information is transmitted in a new regulatory submission or in an amendment, update or supplement to a previous regulatory submission; and
- Whether the document that is the subject of the file exceeds 50 megabytes and, thus, is broken into more than one file.
You should refer to Appendix 12 and the detailed guidance for each type of regulatory submission (in Sections IV through VIII of this document) for more complete recommendations for naming files and for examples.
D. Roadmaps
- What is an electronic submission “roadmap”?
An electronic submission “roadmap” is an organized grouping of electronic folders that you can download and use to structure an electronic submission.
- Do different types of regulatory submissions have different roadmaps?
Yes.
- Should I structure each electronic regulatory submission I make using a roadmap?
Yes. You should structure each electronic regulatory submission (including a new submission and any update, amendment, or supplement to a previous submission) using the applicable roadmap. You should download a new roadmap each time you transmit an amendment, update or supplement to an existing submission. This will help us to distinguish the newly submitted information from the information in your original submission.
- Where can I find the roadmaps for use in structuring regulatory submissions?
The roadmaps are available in Appendix 15.
- Where can I find examples of representative roadmaps for the submissions described in this document?
Figures IV-1, V-1, VI-1, VII-1, and VIII-1 of this document (see Sections IV, V, VI, VII, and VIII of this document), which are submission outlines, depict examples of representative roadmaps for the submissions described in this document.
What would a roadmap look like when displayed on my computer?
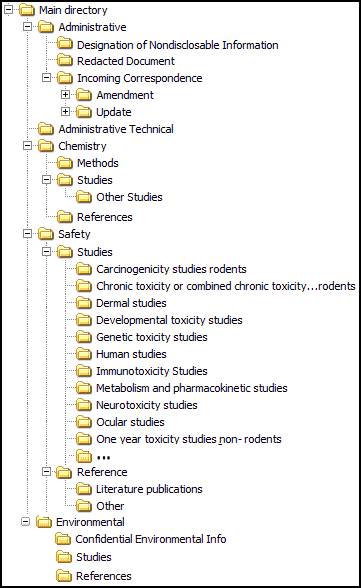
Figures III-1 and III-2 provide examples of a roadmap as seen on your computer (Microsoft Windows). Figure III-1 shows a foldering structure and Figure III-2 shows files included within a particular folder. The information presented in this diagram depicts a set of folders in a food additive petition; an alternative presentation of Figure III-1 (in text format) is in Figure IV-1.
Figure III-1 shows a “Main directory” with “five first level folders” (Administrative, Administrative Technical, Chemistry, Safety and Environmental). The Administrative folder has three second level folders (Designation of Nondisclosable Information, Redacted Document and Incoming Correspondence). The Chemistry folder has two second level folders (Methods and References). The Safety folder has two second level folders (Studies and References). The Environmental folder has three second level folders (Confidential Environmental Info, Studies and References). The second level folders Incoming Correspondence, Chemistry, Safety and Environmental also contain third level folders. The third level Studies folder under the Safety folder has many fourth level folders.
Figure III-1 Example of the Main Directory of a FAP Submission Roadmap
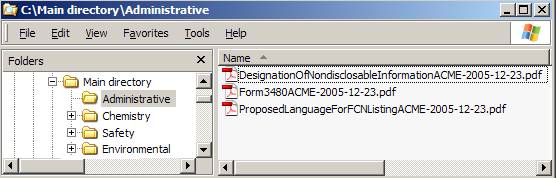
Figure III-2 Example of the Contents of the Administrative Subfolder in a FCN Submission Roadmap
Figure III-2 has two panels. The left panel of Figure III-2 shows a “Main directory” with four “first level folders” (i.e., Administrative, Chemistry, Environmental, and Safety folders). A “plus” sign precedes three folders (i.e., Chemistry, Safety, and Environmental folders), indicating that these folders contain second level folders.
The administrative folder in the left panel of Figure III-2 is highlighted to show that its contents are presented in the right panel of Figure III-2. The right panel of Figure III-2 contains three PDF files (i.e.., for Designation of Nondisclosable Information, Form 3480, and Proposed Language for the FCN Listing for a food contact substance).
E. Organizational Considerations for Files Included in Electronic Regulatory Submissions
- What format features should I include in the Table of Contents in a PDF file included in an electronic regulatory submission?
You should format the Table of Contents in a PDF file using bookmarks designed to help the reader navigate through the document efficiently.
- How should I paginate the individual files in a regulatory submission in electronic format?
You should paginate each individual file transmitted in the regulatory submission. In most cases, you should paginate each submitted file so that it begins on page 1 – i.e., you should not incorporate any type of continuous pagination. This recommendation is in contrast to our recommendation for pagination of paper submissions, where continuous pagination is appropriate (see Section II.D of this document). See the next question for an exception associated with studies that are so large that you submit them in multiple files that use continuous pagination.
- How should I organize information in large studies?
You should organize information in large studies as follows:
- Include a Table of Contents at the beginning of each study;
- Separate files by sections of the study rather than breaking in the middle of a section; and
- Preserve the pagination of a large study with each PDF file continuing the pagination where the previous PDF file left off.
For example, consider a study file with 2000 pages. If you split the file at the end of page 1500, the next file should start at page 1501. (See Appendix 12 for additional information on naming these files.)
- How should I format the Web site addresses included in a reference list submitted in electronic format?
You should format Web site addresses in a reference list as hyperlinks and include the applicable Web site address as of the date when you accessed the reference from the Web site.
F. Preparation of Amendments, Updates, and Supplements in Electronic Format
- What special recommendations apply to the submission of amendments, updates, or supplements in an electronic regulatory submission?
The following recommendations apply to amendments, updates, and supplements for all regulatory submissions prepared in electronic format.
- Use the applicable form.
- Download a new Roadmap and change the name of the “Main directory” to the number we assigned to your submission type (e.g., “FCN 009999” or “FAP 9A9999”).
- Organize the information in the submission into one or more individual files as described in the specific guidance for your regulatory submission. (For example, an amendment or update to a FAP would include a separate file directed to each topic addressed in the amendment or update, whereas an amendment or supplement to a GRAS notice would include a single file regardless of the number of topics addressed in the amendment or supplement.)
- Begin each filename with the date (YYYY-MM-DD) the amendment, update or supplement is submitted.
- Place the files in appropriate folders according to the applicable roadmap.
- Do not include files already submitted.
Additional recommendations for amendments, updates or supplements are available in the specific guidance for each submission type as follows:
- FAPs/CAPs: Section IV.C;
- FCNs: Section V.C;
- GRAS notices: Section VI.C;
- Biotechnology Final Consultations: Section VII.C; and
- New Protein Consultation Submissions: Section VIII.C.
Following these recommendations will help us to store the data and information in the amendment, update or supplement in our database in a way that our reviewers can easily find it.
G. Electronic Signatures
- Where can I find information about electronic signatures in a regulatory submission?
Information about electronic signatures in regulatory submissions to FDA is available in 21 CFR 11.200 and at the ESG (Ref. 1). For further information on the current status of electronic signatures, contact OFAS at 240-402-1200.
Submit Comments
Submit comments on this guidance document electronically via docket ID: FDA-2013-S-0610 - Specific Electronic Submissions Intended For FDA's Dockets Management Staff (i.e., Citizen Petitions, Draft Proposed Guidance Documents, Variances, and other administrative record submissions)
If unable to submit comments online, please mail written comments to:
Dockets Management
Food and Drug Administration
5630 Fishers Lane, Rm 1061
Rockville, MD 20852
All comments should be identified with the title of the guidance.