BAM Chapter 10: Detection of Listeria monocytogenes in Foods and Environmental Samples, and Enumeration of Listeria monocytogenes in Foods
Bacteriological Analytical Manual (BAM) Main Page
Authors: Anthony D. Hitchins (ret.) and Karen Jinneman and Yi Chen
Revision History:
- March 2017: Addition of the sample matrix for environmental samples.
- January 2016: More specific sample preparation and analytical set-up instructions for qualitative detection or quantitative enumeration.
- January 2016: Reorganization and editing of all sections.
- February 2013: update to Table 1; update for BAM Media M52: Buffered Listeria Enrichment Broth (BLEB) in the Media section.
- November 2011: Addition of PCR confirmation for Listeria monocytogenes and Listeria spp. isolates other than L. grayi.
- April 2011: Section E. Diagram describing the Henry Optical System for examination of colonies added; references for Listeria monocytogenes Risk Assessment and Guidance updated.
- August 2002: Section J. Enumeration: Added instructions for positive result on all MPN tubes.
- April 2001: Section H. CAMP test: Updated address for ATCC and added a link to its web page.
The genus Listeria contains 6 species: L. monocytogenes, L. innocua, L. seeligeri, L. welshimeri, L. ivanovii, and L. grayi (Table 1). L. grayi (28, 40) and L. ivanovii (13, 27) each contain two subspecies, which do not need to be specified in this analysis. A taxonomic review of the genus by Rocourt (41) in 1999 updates the previous reviews (11, 43). In recent years, many new species were proposed. However, these new species are not widely adopted and the number of type strains for the newly proposed species are very limited. L. ivanovii and L. monocytogenes are pathogenic for mice and other animals. However, only L. monocytogenes is commonly associated with human listeriosis. Listeriosis associated infection by L. ivanovii, and even by L. seeligeri, is extremely rare in humans. The universal occurrence of L. monocytogenes in food (42) and the risk of contracting foodborne listeriosis (47,48) have been thoroughly reviewed recently. This chapter describes the detection and enumeration of L. monocytogenes in foods and detection from food processing environment.
This standard methodology and alternative rapid methodologies are intended to be used for detection and isolation of L. monocytogenes from foods and environmental samples. Analytical sample size for foods is generally 25 g, and this can be from individual units or as part of a sample composite.
Alternatively, rapid test kits with their respective enrichment media approved as AOAC Official Methods of Analysis (OMA) may be conditionally used to screen for the presence of Listeria contaminants. Putative Listeria isolates on selective agars from standard or screen positive enrichments are purified on non-selective agars and confirmed by conventional identification tests or by a battery of such tests in kit form. Isolates may be rapidly confirmed as L. monocytogenes (or not) by using specific test kits or PCR procedures. Subtyping of L. monocytogenes isolates is generally expected, which includes serological typing and pulsed-field gel electrophoresis (PFGE). Optional pathogenicity testing of L. monocytogenes isolates is described in Section H.
Enumeration of L. monocytogenes in positive food samples is performed on reserve sample by colony count on L. monocytogenes differential selective agars in conjunction with MPN enumeration using selective enrichment in BLEB with subsequent plating on L. monocytogenes differential selective agars as described below.
-
Equipment and materials
FDA does not specifically endorse any of commercial products listed. Equivalent products may be available.
- Sterile swab: the following or equivalent:
- 3M™ Swab-sampler in 10 ml D/E neutralizing broth (Catalog# RS96010DE, www.mmm.com
--]) ) (Polyester).
) (Polyester). - Puritan dry cotton swab (Catalog# 25-806 1PC, 25-806 2PC, www.puritanmedproducts.com) (Cotton)
- World Bioproducts PUR-Blue™ swab sampler with (Catalog #BLU-10DE) or without (Catalog# BLU-DRY, www.worldbioproducts.com
--]) ) 10ml D/E neutralizing broth (Polyurethane).
) 10ml D/E neutralizing broth (Polyurethane). - Healthlink® dry swab transporter (Catalog# 4159BX, www.hardydiagnostics.com
--]) ) (Polyester).
) (Polyester).
- 3M™ Swab-sampler in 10 ml D/E neutralizing broth (Catalog# RS96010DE, www.mmm.com
- Sterile sponge: the following or equivalent:
- World Bioproducts EZ Reach™ sponge sampler with (Catalog# EZ-10DE-PUR) or without (Catalog# EZ-DRY-PUR, www.worldbioproducts.com
--]) ) 10 ml D/E neutralizing broth (Polyurethane).
) 10 ml D/E neutralizing broth (Polyurethane). - Nasco Whirl-Pak® dry sponge probe (Catalog# B01475WA, www.enasco.com
--]) ) (Cellulose).
) (Cellulose). - 3M™ Sponge-sticks with (Catalog# SSL10DE, www.mmm.com
--]) ) or without (Catalog# SSL100) 10 ml D/E neutralizing broth (Cellulose).
) or without (Catalog# SSL100) 10 ml D/E neutralizing broth (Cellulose).
If available, dry samplers can be obtained and D/E neutralizing broth added later, or pre-moistened samplers can be obtained. We do not recommend less than 10 ml D/E broth due to the possible presence of sanitizer residues on environmental surfaces.
- World Bioproducts EZ Reach™ sponge sampler with (Catalog# EZ-10DE-PUR) or without (Catalog# EZ-DRY-PUR, www.worldbioproducts.com
- Balance for weighing sample to 0.1 g
- Incubators, 30 and 35°C
- Water bath, 80°C ± 2°C
- Phase-contrast microscope with oil immersion phase objective (100×)
- Blender motor and jars or Stomacher and bags
- Vortex mixer
- Sterile swab: the following or equivalent:
-
FDA does not specifically endorse any of commercial products listed. Equivalent products may be available.
- Buffered Listeria Enrichment Broth (BLEB) (M52)
- Acriflavine monohydrochloride
- Nalidixic acid (sodium salt)
- Cycloheximide
- Natamycin (Pimaricin)
- Dey/Engley (D/E) broth (M193)
- Oxford medium (OXA) (M118)
- PALCAM agar (M118a)
- MOX agar (M103a)
- Lithium chloride-phenylethanol-moxalactam (LPM) agar (M81) with added esculin and iron (M82)
- R&F Listeria monocytogenes Chromogenic Plating Medium (R&F Laboratories, Downers Grove, IL) (M17a)
- ALOA agar (M10a)
- Chromogenic Listeria Agar (Oxoid Ltd, Basingstoke, England) (M40b)
- Rapid’ L.mono (BioRad Laboratories Inc.) (M131a)
- CHROMagar Listeria (CHROMAgar, Paris, France) (M40a)
- Trypticase soy agar with 0.6% yeast extract (TSAYE) (M153)
- Sheep Blood Agar (M135)
- Hydrogen peroxide solution, 3% for catalase test (R12)
- Gram stain kit
- Motility test medium (MTM, Difco) (M103)
- Trypticase soy broth with 0.6% yeast extract (TSBye) (M157)
- Purple carbohydrate fermentation broth base (M130), containing 0.5% solutions of dextrose, esculin, maltose, rhamnose, mannitol, and xylose
- Physiological saline solution, 0.85% (R63)
- Fluorescent antibody (FA) buffer (Difco)
- Listeria-typing sera Type 1 (Difco cat. # 223031) and Type 4 (Difco cat. # 223041)
- Listeria Antisera Set (Denka Seiken product #294616)
- Optional: Nitrate reduction medium (M108) and nitrate detection reagents (R48)
Note: Alternative companies may be used when the products are equivalent.
-
Control Cultures
- Listeria monocytogenes ATCC 19115
- Listeria innocua ATCC 33090
- Listeria seeligeri ATCC 35967
- Listeria ivanovii ATCC 19119
- Rhodococcus equi ATCC 6930
- Staphylococcus aureus ATCC 25923 or ATCC 49444
-
Sample Preparation
Sample transport and storage practices should maintain the recommended storage conditions for the food commodity. Sample analysis should be initiated as soon as possible upon sample receipt. If sample analysis must be delayed, store frozen samples frozen (-20 °C ± 5 °C); store nonperishable, canned or low-moisture foods at room temperature, and store refrigerated, unfrozen perishable foods at 4 °C ± 2 °C until sample analysis is initiated.
Basic analytical options include: 1. Qualitative detection (limit of detection <1 cfu="" per="" analytical="" unit),="" 2.="" quantitative="">
- Qualitative detection from foods and environmental samples
- Individual subsample analysis: For solids, semi-solids, or liquids add 25g representative portion to 225 ml BLEB containing pyruvate without selective additives (M52). (10, 26). Thoroughly mix, blend or stomach, continue enrichment as described in section E or F. Certain foods may require different sample set-up procedures such as soaking and rinsing. A 50 g portion of the sample should be reserved for possible pathogen enumeration. Store it at 5° C if it is not frozen or, if frozen, in a non-defrosting freezer. Refer to applicable sampling compliance guidance documents for additional instructions.
- Composite sample analysis: composites may be used to analyze multiple sub units from a single sample. Refer to applicable sampling compliance guidance documents for specific composite procedures. Generally, two composites are prepared from a sample consisting of 10 sub-samples (liquid, cream or solid food). To prepare a composite, 50-g or ml representative portions from each of the 5 sub-samples are pooled and 250 ml basal BLEB without selective agents is added and then blended or stomached (M52). A 50 g portion of this composite blend (equivalent to 25g food plus 25 ml basal BLEB) is combined with 200 ml of basal BLEB. The composite is then inoculated as described in section E or F. An aliquot (100 ml) of the composite blend should be retained, preferably at 5° C and not below 0° C, for possible pathogen enumeration.
- Environmental samples: When sampling dry surfaces, swabs (cotton, polyester or polyurethane) or sponges (cellulose or polyurethane) should be pre-moistened in 10 ml Dey/Engley (D/E) neutralizing broth. Swabs are typically stored in tube containers and sponges are typically stored in bag containers. Before sampling, press swabs against inner tube walls or squeeze the sponges in bags to remove excess broth. Swab environmental surfaces using firm and even pressure vertically (approximately 10 times), then flip the sampler and use the other side to swab horizontally (approximately 10 times) and diagonally (approximately 10 times). When putting the samplers back to the container, make sure they are submerged in the D/E neutralizing broth. When sampling wet surfaces, dry swabs or sponges should be used and put into D/E neutralizing broth immediately after sampling. Swabs that are made from cotton or that have large tips appear to absorb liquid better than others. For samples that are not suitable to be collected by regular sized swabs/sponges mentioned in section A, refer to applicable sampling compliance guidance documents for additional instructions. After sampling, swab/sponge can be maintained in D/E broth at 4°C for up to 48 h before analysis. Swab/sponge and D/E neutralizing broth are then added to 90 ml (or more to fully submerge the swab or sponge) of BLEB containing pyruvate without selective additives (M52). Sponge and D/E broth can also be added to 225ml of BLEB. Thoroughly massage or stomach to expel the collection broth into enrichment broth. Continue enrichment as described in section E or F.
- Interpret result. Confirmation of one or more L. monocytogenes isolates from an enrichment indicates that L. monocytogenes is present at ≥ 1 CFU per sample size analyzed or present on environmental swab or sponge sample.
- Quantitative determination from foods. Enumeration is performed using a combination of MPN and direct plating. Refer to section J for details. Surveillance samples are generally first analyzed by qualitative detection and reserve portions of the positive samples are then enumerated. For outbreak response situations, all samples may be directly enumerated. Refer to section J and applicable sampling compliance guidance documents.
- Qualitative detection from foods and environmental samples
- Enrichment Procedure
- Incubate food samples or environmental samples homogenized in BLEB with pyruvate (M52, 43) at 30° C, for 4 h.
- Aseptically add the three filter sterilized selective agents (M52) to achieve final concentrations of 10 mg/L acriflavin, 40 mg/L cycloheximide and 50 mg/L sodium nalidixic acid in the BLEB with pyruvate pre-enrichments.
- Mix the enrichment with additives and continue incubation at 30° C for the remainder of the 24 to 48 h enrichment period.
- Alternate Screening Methodologies
The following alternative screening methodologies may be used to screen samples for the presence of Listeria. Follow the manufacturers' package insert making certain they have not deviated from the approved versions of the AOAC INTERNATIONAL Official Methods Manual protocols (Section F1). The kits are only approved for the specified food matrices, claimed in the OMA method, which vary from kit to kit. For other food matrices that are not validated a matrix extension validation is necessary. Negative results obtained with the products are considered definitive and no further testing is required. Presumptive positive results with these rapid screening methods must be confirmed by streaking to the selective agars and confirming isolates to the species level by the procedures described in sections G-I.
F1. Rapid screening methods for Listeria spp.
F2. Rapid screening methods for Listeria monocytogenes. Please note that these methods do not screen for Listeria spp. and therefore may not be suitable for situations in which the identification of Listeria spp. is desired.
-
- AOAC Official Method 993.09. Listeria in select foods Colorimetric deoxyribonucleic acid hybridization method (GENE-TRAK Listeria Assay). (3, 16)
(Applicable to dairy products, meats, and seafoods)
Collaborative study: milk (2%), brie cheese, cooked crab meat, frankfurters, roast beef, raw ground pork
Pre-collaborative: crab meat, raw shrimp, cheddar cheese, cottage cheese, ice cream, chocolate milk, nonfat dried milk, fish fillet, ground raw pork, fermented sausage, raw ground turkey.
-
AOAC Official Method 994.03. Listeria in select foods. Colorimetric Monoclonal Enzyme-Linked Immunosorbent Assay Method (Listeria-Tek) (4, 17, 31)
(Applicable to dairy products, seafoods, meats)
Collaborative study: frankfurters, roast beef vacuum packed, brie cheese, 2% milk, raw frozen shelled shrimp, cooked frozen crab.
Pre-collaborative: crab meat, ice cream, milk, chocolate milk, non-fat dried milk, raw fish, cooked beef, roast beef, cured ham, raw sausage, raw oyster, raw chicken, raw turkey.
- AOAC Official Method 995.22. 2000. Listeria in select foods. Colorimetric polyclonal enzyme immunoassay screening method (TECRA Listeria Visual Immunoassay). (5, 29)
(Applicable to dairy foods, seafoods, poultry, meats (not raw ground chuck), leafy vegetables)
Collaborative study: fish fillets, ice cream, lettuce, chicken, ground turkey
Pre-collaborative: crabmeat, shrimp, soft cheese, chocolate milk, non-fat dried milk, raw beef, roast beef, frankfurters, bologna, oysters, chicken.
- AOAC Official Method 2002.09. Listeria in select foods. TECRA Listeria Visual
Immunoassay Using TECRA Listeria Enrichment Broth. (32, 5, 29).
(Applicable to raw and processed meats, cultured and non-cultured dairy products);
Note: Method is based upon 995.22 but with altered enrichment, omission of cyclohexamide and additional foods.
Collaborative study: fish fillets, turkey, raw ground beef, ice cream, lettuce.
- AOAC Official Method 996.14. Listeria in select foods Assurance Polyclonal Enzyme Immunoassay Method (EIA) (6, 19).
(Applicable to dairy foods, red meats, pork, poultry products, fruits, nutmeats, seafood, pasta, vegetables, cheese, animal meal, chocolate, and eggs, bone meal and from environmental surfaces)
Collaborative study: nonfat dried milk, ice cream, raw poultry, raw shrimp, cooked roast beef, green beans
Pre-collaborative: crabmeat, soft cheese, dry egg, egg liquid frozen, milk, chocolate milk, raw fish, bone meal, raw beef, raw pork, scallops, chocolate, nuts, pasta, raw chicken, coleslaw
- AOAC Official Method 997.03. Listeria in select foods Visual Immunoprecipitate Assay (VIP) (7, 20).
(Applicable to dairy foods, red meats, pork, poultry and poultry products, seafood, fruits, vegetables, nutmeats, pasta, chocolate,eggs, and bone meal, and environmental surfaces.
Collaborative study: nonfat dried milk. Ice cream, raw poultry, raw shrimp, cooked roast beef, green beans, environmental surfaces
- AOAC Official Method 999.06. Listeria in select foods. Enzyme Linked Immunofluorescent Assay (ELFA) VIDAS LIS Assay Screening Method (8, 21).
(Applicable to dairy products, vegetables, seafoods, raw meats and poultry, and processed meats and poultry)
Collaborative study: ice cream, green beans, fish, turkey, cheese, roast beef
- AOAC Official Method 2004.06. Listeria in select foods. Modified VIDAS LIS Assay Screening Method (36).
(Applicable to the detection of Listeria in dairy products, vegetables, seafood, raw meats and poultry, and processed meats and poultry.)
Collaborative study: brie cheese, ice cream, fish, green beans, roast beef
- AOAC Official Method 2010.02. Listeria in select foods. VIDAS LSX Assay Screening Method (35).
(Applicable to dairy products, vegetables, seafood, raw meats and poultry, and processed meats and poultry.)
Collaborative study: vanilla ice cream, cheddar cheese, raw ground beef, frozen green beans, deli turkey, cooked shrimp
- AOAC Official Method 2013.10. Listeria in select foods and environmental surfaces. VIDAS UP Listeria (LPT) Assay Kit (36).
(Applicable to deli ham (25 and 125 g), pepperoni (25 g), beef hot dogs (25 g), chicken nuggets (25 g), chicken liver pâté (25 g), ground beef (125 g), deli turkey (125 g), cooked shrimp (25 g), smoked salmon (25 g), whole cantaloupe melon, bagged mixed salad (25 g), peanut butter (25 g), black pepper (25 g), vanilla ice cream (25 g), queso fresco (25 and 125 g), stainless steel, plastic, ceramic and concrete environmental surfaces.)
See supplemental data, Tables 2A–D, for detailed results of the collaborative study on J. AOAC Int. website, http://aoac.publisher.ingentaconnect.com/content/aoac/jaoac.
- AOAC Official Method 2003.12. Listeria monocytogenes in select foods. BAX® Automated System (9).
(Applicable to dairy products, fruits and vegetables [except radishes], seafoods, raw and processed meats, and poultry.)
Collaborative study: frankfurters, soft cheese, smoked salmon, ground beef, radishes, peas
- AOAC Official Method 2004.02. Listeria monocytogenes in select foods. Enzyme Linked Immunofluorescent Assay (ELFA) VIDAS LMO2 Assay Screening Method (33).
(Applicable to the detection of L. monocytogenes in dairy products, vegetables, seafood, raw meats and poultry, and processed meats and poultry.) Collaborative study: vanilla ice cream, brie cheese, coked roast beef, frozen green beans, frozen tilapia fish
- AOAC Official Method 2013.11. Listeria monocytogenes in select foods. VIDAS Listeria monocytogenes (LMX) Assay Kit (37).
(Applicable to deli ham (25 and 125 g), fermented sausage (25 g), liver pâté (25 g), processed cheese (25 g), vanilla ice cream (25 g), cooked shrimp (25 g), smoked white fish (25 g), frozen spinach (25 g), peanut butter (25 g), deli turkey (25 and 125 g), queso fresco (125 g), and ground beef (125 g).)
- AOAC Official Method 993.09. Listeria in select foods Colorimetric deoxyribonucleic acid hybridization method (GENE-TRAK Listeria Assay). (3, 16)
-
- Isolation Procedure
-
At 24 and 48 h, streak BLEB enrichments onto one esculin-based and one chromogenic selective agar from each of the categories listed in sections G.1.A and G.1.B. Incubate plates for up to 48 h. Check plates at both 24 h and 48h.
-
Esculin based Listeria selective agars:
- Oxford agar (OXA) (18) ( M118). After 24 h incubation at 35° C typical Listeria species colonies are approximately 1 mm diameter, gray to black colonies surrounded by a black halo. Following 48 h incubation typical Listeria species colonies are approximately 2-3 mm diameter, black with a black halo and sunken center.
- PALCAM (50) (M138a). Incubation conditions and appearance of Listeria species colonies are the same as for Oxford agar except that the background plate color is red.
- Modified Oxford Agar (MOX) (46) ( M103a). Incubation conditions and appearance of Listeria species colonies are the same as for Oxford agar.
- LPM (30) ( M81) fortified with Esculin and Fe3+. Incubate at 30° C. Typical Listeria species colony appearance is similar to Oxford agar.
-
Chromogenic L. monocytogenes-L. ivanovii chromogenic agars:
- R&F Listeria monocytogenes Chromogenic Plating Medium (R&F LMCPM) (39, 25) (M17a) Incubate plates at 35° C, L. monocytogenes and L. ivanovii produce a 1-3 mm diameter, smooth, convex, blue/green colony and small blue/green halo. All other Listeria species produce a 1-2 mm, smooth, convex white colony with no halo. Although rare, L. ivanovii have been isolated from foods previously. Typical L. monocytogenes and L. ivanovii colonies can be distinguished using a commercial Confirmatory Medium (R&F laboratories) or by the identification methods described in section H.
- RAPID’ L. mono (M131a): Incubate plates at 37° C, L. monocytogenes and L. ivanovii produce a 1-3 mm diameter, smooth, convex, blue/green colony. Typical colonies appear dark blue/green in the red background of RAPID’ L. mono agar and appear blue/green when the background flora change the background color of the agar to yellow. Additionally a yellow halo will surround L. ivanovii colonies. However, yellow halo may be obvious in areas of at least moderate growth. Heavy growth of L. ivanovii could turn the entire plate to yellow. In addition, caution has to be taken for L. monocytogenes-L. ivanovii differentiation because background flora in some food commodities can change the color of certain areas of this agar to yellow, and this could make L. monocytogenes appear as L. ivanovii. All other Listeria species produce a 1-2 mm, smooth, convex white colony with or without a yellow halo.
- Agar Listeria according to Ottaviani and Agosti (ALOA) (44, 49) (M10a), or Oxoid Chromogenic Listeria agar (OCLA) (M40b). Incubate plates at 37 °C, all Listeria species appear as 1-3 mm diameter blue/green colonies. Additionally, L. monocytogenes and L. ivanovii have an opaque white halo surrounding the colony.
- CHROMagar Listeria (1) (M40a) – Incubation conditions and appearance of Listeria colonies are the same as for ALOA except that the background plate color is light blue.
For the approved rapid methods, use the selective isolation agar recommended by the manufacturer but auxiliary use of chromogenic L. monocytogenes-L. ivanovii differential agars is also recommended.
Note: The chromogen used in both the R&F LMCPM and RAPID’ L. mono agars is indicative of phosphoatidylinositol-specific phospholipase C (PI-PLC) activity. On these agars Listeria species with PI-PLC activity, L. monocytogenes and L. ivanovii, will appear blue-green and all other Listeria species will not develop the blue-green color and remain white in appearance. In the case of ALOA and CHROMagar the presence of a Listeria species based on a specific β-glucosiadase enzyme activity which is detected by the chromogen, therefore, all Listeria species will appear blue-green on these agars. The phospoholipase activity specific for L. monocytogenes and L. ivanovii is determined by the additional opaque white halo surrounding the colony.
For the approved rapid methods, use the selective isolation agar recommended by the manufacturer but auxiliary use of chromogenic L. monocytogenes-L. ivanovii differential agars is also recommended.
-
- Select up to 5 typical colonies from each esculin based agar and streak for purity to TSAYE (M153) and incubate plates at 30° C for 24-48 h. Select up to 2 typical colonies for streaking if using L. monocytogenes-L. ivanovii differential chromogenic agars. The plates may be incubated at 35° C if colonies will not be used for wet-mount motility observations.
- If isolated colonies are available use remaining colony growth to stab a 5% sheep blood agar (M135) plate. Incubate at 35 °C for 24-48 h.
-
- Identification Procedure
Identify purified isolates from growth on the TSAYE plate by the following classical tests (H.1.a-e). Alternatively, rapid biochemical test kits or PCR analyses may be used to confirm isolates to the species level (section H.2.a-c).
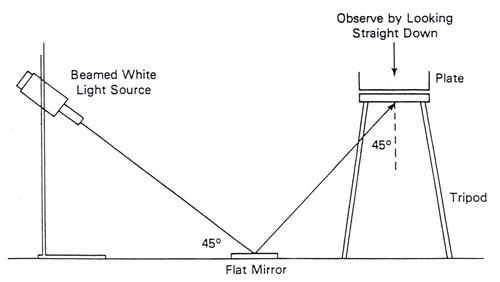
Examine TSAYE plates for typical 1-3 mm diameter smooth convex white colonies. Observation with Henry oblique transmitted illumination (23) can be helpful at this stage but is not mandatory.
Figure 1. Henry Optical System for Examination of Colonies
- Standard Classification
- Hemolysis:
Inoculate heavily (from TSAye colony) 5% sheep blood agar by stabbing plates that have been poured thick and dried well (check for moisture before using). Draw grid of 20-25 spaces on plate bottom. Stab one culture per grid space. Always stab positive controls (L. ivanovii and L. monocytogenes) and negative control (L. innocua). Incubate for 24-48 h at 35° C. Attempt to stab as near to bottom of agar layer as possible, without actually touching bottom of agar layer and possibly fracturing the agar.
Examine blood agar plates containing culture stabs brightly lit from behind the plate. L. monocytogenes and L. seeligeri produce a slightly cleared zone around the stab. L. innocua shows no zone of hemolysis, whereas L. ivanovii produces a well-defined clear zone around the stab. If mixed culture was observed on the TSAYE plate repeat the hemolysis test with an isolated colony.
CAMP test: Resolve questionable reactions by the Christie-Atkins-Munch-Peterson (CAMP) (15) test. CAMP test strains are available from culture collections, including the American Type Culture Collection (ATCC), Manassas, VA, http://www.atcc.org
- Streak weakly β-hemolytic S. aureus (FDA strain ATCC 49444 (CIP 5710; NCTC 7428) or ATCC 25923) and R. equi (ATCC 6939; NCTC 1621) vertically on sheep blood agar.
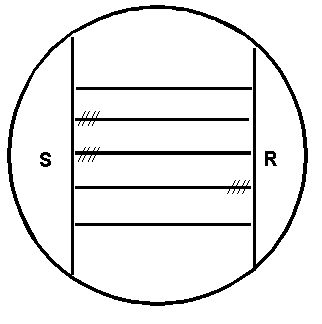
- Separately streak test strains horizontally between the S. aureus and R. equi streaks without quite touching them. Incubate plate 24 to 48-h at 35° C. Figure 1 shows the arrangement of the culture streaks on a CAMP plate.
- Examine plates for hemolysis in the zone of influence of the vertical streaks. Hemolysis of L. monocytogenes and L. seeligeri is enhanced near the S. aureus streak; L. ivanovii hemolysis is enhanced near the R. equi streak. Other species are non-hemolytic and do not react in this test (Table 1).
- Alternatively, a factor easily prepared from S. aureus cultures can be used to enhance hemolysis by L. monocytogenes and L. seeligeri in sheep blood agar plates. Disks impregnated with the β-lysin of S. aureus (REMEL, Lenexa, KS).
Table 1. Differentiation of Listeria speciesSpecies Mannitol Rhamnose Xylose Virulencea β-Hemolysisb Hemolysis enhancement with
Staphylococcus aureus (S)Hemolysis enhancement with
Rhodococcus equi (R)L. monocytogenes - +c - + + + -d L. ivanoviie - - + + + - + L. innocua - Vf - - - - - L. welshimeri - V + - - - - L. seeligeri - - + - +g + - L. grayih + V - - - a Mouse Test
b Sheep blood agar stab
c Some lineage III strains of L. monocytogenes, which are primarily associated with animal listeriosis, are rhamnose negative.
d Rare strains are S+ and R+. The R+ reaction is less pronounced than that of L. ivanovii.
e Ribose fermenting strains are classified as L. ivanovii subsp. ivanovii and ribose non-fermenters as L. ivanovii subsp. Londiniensis.
f V, variable biotypes, greater than 10% of strains for this trait.
g Weakly hemolytic L. seeligeri strains may appear non hemolytic.
h Includes two subspecies - L. grayi subsp. murrayi reduces nitrate L. grayi subsp. grayi does not reduce nitrate.Figure 2. CAMP test for Listeria monocytogenes: Inoculation pattern of the sheep blood agar plate. Horizontal lines represent streak inoculations of 5 test strains. Vertical lines represent streak inoculations of Staphylococcus aureus (S) and Rhodococcus equi (R). Hatched lines indicate (diagrammatically only) the locations of hemolysis enhancement regions.
- Motility: Pick typical colony from TSAYE and examine by wet mount, using 0.85% saline for suspending medium and oil immersion objective of phase-contrast microscope. Choose a colony with enough growth to make a fairly heavy suspension; emulsify thoroughly. Listeria spp. are slim, short rods with slight rotating or tumbling motility. Always compare with known culture. Cocci, large rods, or rods with rapid, swimming motility are not Listeria spp. Alternatively, stab tube of MTM (M103) from TSAYE. Incubate for up to 7 days at room temperature (20-25° C). Observe daily until the isolate growth pattern is obvious. Listeria is motile, giving a typical umbrella-like growth pattern.
- Catalase: Test typical colonies for catalase by placing some colony growth in a drop of 3% hydrogen peroxide. Listeria species are catalase-positive.
- Gram stain: Use 16- to 24-h growth from TSAYE plates. All Listeria spp. are short, Gram-positive rods; however, with older cultures the Gram stain reaction can be variable and also cells may appear spheroidal. The cells have a tendency to palisade in thick-stained smears. This can lead to false rejection as a diphtheroid.
- Carbohydrate fermentation series. Pick typical colony to a tube of TSBYE for inoculating carbohydrate fermentation and other test media. Incubate at 35° C for 24 h. This culture may be kept at 4°C several days and used repeatedly as inoculum.
- From TSBYE culture, inoculate the following carbohydrates in 0.5% solutions in purple carbohydrate broth with Durham tubes: dextrose, esculin, maltose, rhamnose, mannitol, and xylose. Incubate 7 days at 35° C.
- Positive reactions will be indicated by the production of acid and the media turning a yellow color with no gas production. All Listeria species should be positive for dextrose, esculin, and maltose. All Listeria spp. except L. grayi should be mannitol-negative. If pigmentation of the isolate on OXA, PALCAM, MOX or LPM plus esculin/Fe3+ is unequivocal, the esculin test may be omitted. Consult Table 1 for interpretations of the results.
- Optional: Nitrate reduction test: Only L. grayi ssp. murrayi reduces nitrates. The test distinguishes L. grayi ssp. murrayi from L. grayi ssp. grayi.
- Use a TSAYE culture to inoculate nitrate broth (M108). Incubate at 35° C for 5 days.
- Add 0.2 ml reagent A, followed by 0.2 ml reagent B (R48). A red-violet color indicates presence of nitrite, i.e. nitrate has been reduced. If no color develops, add powdered zinc and hold for 1 h. A developing red-violet color indicates that nitrate is still present and has not been reduced.
- As an alternative procedure, add 0.2 ml reagent A followed by 0.2 ml reagent C. An orange color indicates reduction of nitrate. If no color develops, add powdered zinc as above. Development of an orange color indicates unreduced nitrate.
- Optional: Since all Listeria species test negative for indole, oxidase, urease, and H2S production from organic sulfur compounds (H2S is produced from thiosulfate in the MICRO-ID test kit) and test positive for methyl red and Voges-Proskauer, these tests are discretionary. Brochothrix, which is phylogenetically closely related to Listeria, is distinguishable from Listeria by its inability to grow at 35° C and by its lack of motility. Distinguishing features of the Gram-positive non-sporeforming rods, Erysipelothrix and Kurthia, which occur rarely in Listeria analysis, can be found elsewhere (11, 43).
- Optional: Immunocompromised mouse pathogenicity test: The classical tests for Listeria pathogenicity are the Anton conjunctivitis test (rabbits), inoculation of mice, and inoculation of embryonated eggs. The immunocompromised mouse test, using intra-peritoneal (i.p.) injection, is recommended because of its greatly improved sensitivity (38). Confirmation of L. monocytogenes animal pathogenicity is not needed for clinical isolates and is optional for food isolates. An isolate should be identified as L. monocytogenes if it meets all the other criteria outlined in this chapter. See link for detailed protocol.
Biochemical and pathogenicity data are summarized in Table 1. All data collection must be completed before species identities are determined and subsequent subtyping performed. Atypical Listeria strains exist that could confuse the identification. For example, there are undocumented references (46) to hemolytic L. innocua isolates. Some L. monocytogenes and L. welshimeri isolates are rhamnose-negative. Some L. seeligeri isolates have very weak hemolytic reaction and can be confused with non-hemolytic species. Sometimes aberrant Listeria strains are isolated which are extremely difficult to speciate (26). (See Guideline for BAM Users on Identification of Atypical Hemolytic Listeria Isolates.) If such an aberrant Listeria isolate is obtained, contact Karen Jinneman.
- Hemolysis:
- Alternate rapid identification
Purified isolates may be rapidly identified by using commercial kits or real-time PCR. Follow manufacturer instructions for inoculation and interpretation.
- API Listeria (bioMerieux, Durham, NC) which requires an additional β-hemolysis test for final isolate identification (12). CAMP test is optional.
- Micro-ID Listeria Identification System (Remel, Lenexa, KS) which requires an additional CAMP test and β-hemolysis test. (2, 22).
- Vitek 2 Automatic Gram Positive card (bioMerieux, Hazelwood, MO), which requires an additional CAMP test and β-hemolysis test (38).
- Real-time PCR, which requires an additional β-hemolysis test. CAMP test is optional.
- Protocol: Simultaneous Confirmation of Listeria species and L. monocytogenes isolates by real-time PCR.
- Attachment 1: Single Lab Validation (SLV) - Individual Ct values for each isolate by each enzyme mix. (pdf, 58Kb)
- Attachment 2: Multi Lab validation (MLV) - Individual Ct values for each isolate by laboratory. (pdf, 60Kb)
- Listeria species identification includes L. monocytogenes, L. innocua, L. ivanovii, L. seeligeri and L. welshimeri. Identification of L. grayi has not been verified with this assay. Isolates that are identified as Listeria species but not L. monocytogenes can be fully speciated as described in steps H1.a-e or H2. a-c.
Alternative AOAC OMA rapid methods for the detection of L. monocytogene listed in section F2 can be used to confirm pure culture. Depending on the kit, isolates may be identified in pure culture or from OXA or the other selective isolation agars. Purified isolates identified as Listeria monocytogenes by these tests should be retained for regulatory reference.
For environmental samples, refer to applicable sampling compliance guidance documents to determine if identification to the genus level is sufficient and if further differentiation between L. monocytogenes and other Listeria species is necessary.
- Standard Classification
- Subtyping of L. monocytogenes isolates (required)
Confirmed L. monocytogenes isolates should be typed serologically and genetically.
-
Serological typing. Serology is useful when addressing epidemiological considerations. Most L. monocytogenes isolates obtained from patients, foods, and the environment are type 1 or 4. More than 90% of L. monocytogenes isolates can be serotyped with commercially available sera. However, all nonpathogenic Listeria species, except L. welshimeri, share one or more somatic antigens with L. monocytogenes (43). Therefore, serotyping alone without thorough isolate characterization is not adequate for identification of L. monocytogenes.
Use commercial sera (Difco Type 1 cat #223031 and Type 4 cat #223041) to characterize isolates as type 1, type 4 or not type 1 or 4 (types 3, 5, 6 etc.) at a minimum. Use a TSBye culture to inoculate Tryptose broth. Incubate for 24 h at 35° C, at which temperature flagella (H) antigen expression is reduced. Transfer to Tryptose agar slants and incubate for 24 h at 35° C. Wash both slants in a total of 3 ml Difco fluorescent antibody (FA) buffer and transfer to a sterile 16 × 125-mm screw-cap tube. Heat in a water bath at 80°C for 1 h. Sediment cells by centrifugation at 1600 g for 30 min. Remove 2.2-2.3 ml of supernatant fluid and resuspend the pellet in the remainder of buffer. Follow manufacturer's recommendations for sera dilution and agglutination.
Complete serological characterization can also be done (Denka Seiken product #294616). Pure cultures of L. monocytogenes isolates should be cultured for 24 h at 35° C on non-selective agar such as BHI agar. Colony growth is then resuspended, heat inactivated and tested for agglutination as recommended by the antisera manufacturer.
- Genetic subtyping. Pulsed-field gel electrophoresis (PFGE) by standard PulseNet protocols of food and environmenal isolates should be submitted to PulseNet (CDC, Atlanta, GA). Retain all isolates as additional subtyping techniques may also be requested.
-
- Enumeration (required)
If a food sample tests positive for L. monocytogenes, use a reserve portion of sample for enumeration. Enumeration is performed using a combination of MPN and direct plating.
MPN procedure:
- Prepare a homogenate of a 25-g amount of reserve food sample in 225 ml pre-warmed BLEB with or without pyruvate and without delayed addition of selective agents).
- Prepare a 4-dilution, 3-tube MPN using dilutions that will deliver equivalent to 10, 1, 0.1, and 0.01g sample per aliquot at each respective dilution.
- Incubate all twelve aliquots as described in section E.
- At 48 h streak each aliquot as described in section G followed by isolation and confirmation according to sections G-H. Alternatively each of the enrichments can be rapidly screened by one of the approved methods described in section F with confirmation of all presumptive positives by the isolation and confirmation steps as described in sections G-H.
- Interpret results based on the number of tubes with confirmed positive L. monocytogenes using the tables in BAM Appendix 2 (14).
- If all the MPN tubes are Listeria positive, refer to direct plating for enumeration results.
- In situations that would require narrower confidence limits, the number of replicate tubes for certain dilutions could be increased. One ml of homogenate of complete BLEB and sample can be added and diluted by multi-channel pipette or robotically, in 96-well plates. If the number of tubes are above 3 or the number of tubes are not equal among different dilutions, an AOAC validated MPN calculator can be used: (http://www.aoac.org/imis15_prod/Programs/07trad02LCFMPNCalculator.zip)
Direct plating procedure:
- For solid food, prepare a homogenate of a 25-g amount of reserve food sample in 225 ml BLEB without pyruvate or selective agents. Perform another 10 fold dilution. Certain foods may require different sample set-up and dilution procedures, refer to applicable compliance assignment documents.
- Direct plate 1 ml of liquid food or 1 ml homogenate of solid food prepared in step a onto 3 to 5 plates of one of the L. monocytogenes differential chromogenic agars as described in section G.
- If the colonies on the plates are too numerous to count, use reserve sample, perform additional 10 fold serial dilutions and proceed with direct plating.
- Confirm 5 representative colonies per plate.
- Alternatively, spiral plating method described in Chapter 3 can be used for direct plating.
Notes: Guideline for BAM Users on Identification of Atypical Hemolytic Listeria Isolates
References
- Anonymous. 2001. Validation certificate for alternative method according to the standard EN ISO 16140:2003. http://www.chromagar.com/fichiers/1450365640CHR_21_01_12_01_en_V2013.pdf
- AOAC Official Method 992.18. 2000. MICRO-ID Listeria. Chapter 17.10.02, pp. 141-144 In: Official Methods of Analysis of AOAC INTERNATIONAL. 17th Edition. W. Horwitz (ed.). Volume 1. Agricultural Chemicals, Contaminants and Drugs. AOAC INTERNATIONAL, Gaithersburg, MD.
- AOAC Official Method 993.09. 2000. Listeria in dairy products, seafoods, and meats. Colorimetric deoxyribonucleic acid hybridization method (GENE-TRAK Listeria Assay). Chapter 17.10.04, pp. 147-150 In: Official Methods of Analysis of AOAC INTERNATIONAL. 17th Edition. W. Horwitz (ed.). Volume 1. Agricultural Chemicals, Contaminants and Drugs. AOAC INTERNATIONAL, Gaithersburg, MD.
- AOAC Official Method 994.03. 2000. Listeria monocytogenes in dairy products, seafoods, and meats. Colorimetric monoclonal enzyme-linked immunosorbent assay method (Listeria-Tek). Chapter 17.10.05, pp. 150-152 In: Official Methods of Analysis of AOAC INTERNATIONAL. 17th Edition. W. Horwitz (ed.). Volume 1. Agricultural Chemicals, Contaminants and Drugs. AOAC INTERNATIONAL, Gaithersburg, MD.
- AOAC Official Method 995.22. 2000. Listeria in foods. Colorimetric polyclonal enzyme immunoassay screening method (TECRA Listeria Visual Immunoassay [TLVIA]). Chapter 17.10.06, pp. 152-155 In: Official Methods of Analysis of AOAC INTERNATIONAL. 17th Edition. W. Horwitz (ed.). Volume 1. Agricultural Chemicals, Contaminants and Drugs. AOAC INTERNATIONAL, Gaithersburg, MD.
- AOAC Official Method 996.14. 2000. Assurance Polyclonal Enzyme Immunoassay Method. Chapter 17.10.07, pp. 155-158 In: Official Methods of Analysis of AOAC INTERNATIONAL. 17th Edition. W. Horwitz (ed.). Volume 1. Agricultural Chemicals, Contaminants and Drugs. AOAC INTERNATIONAL, Gaithersburg, MD.
- AOAC Official Method 997.03. 2000. Visual Immunoprecipitate Assay (VIP). Chapter 17.10.08, pp. 158-160 In: Official Methods of Analysis of AOAC INTERNATIONAL. 17th Edition. W. Horwitz (ed.). Volume 1. Agricultural Chemicals, Contaminants and Drugs. AOAC INTERNATIONAL, Gaithersburg, MD.
- AOAC Official Method 999.06. 2000. Enzyme Linked Immunofluorescent Assay (ELFA) VIDAS LIS Assay Screening Method. Chapter 17.10.09, pp. 160-163. In: Official Methods of Analysis of AOAC INTERNATIONAL. 17th Edition. W. Horwitz (ed.). Volume 1. Agricultural Chemicals, Contaminants and Drugs. AOAC INTERNATIONAL, Gaithersburg, MD.
- AOAC Official Method 2003.12. 2005. Evaluation of BAX® Automated System for the Detection of Listeria monocytogenes in Foods. Chapter 17.10.10, pp. 222-225. In: Official Methods of Analysis of AOAC INTERNATIONAL. 18th Edition. W. Horwitz (ed.). AOAC INTERNATIONAL, Gaithersburg, MD.
- Asperger, H., H. Heistinger, M. Wagner, A. Lehner and E. Brandl. 1999. A contribution of Listeria enrichment methodology - growth of Listeria monocytogenes under varying conditions concerning enrichment broth composition, cheese matrices and competing microflora. Microbiology 16:419-431.
- Bille, J., J. Rocourt, and B. Swaminathan. 1999. Listeriae, Erysipelothrix, and Kurthia, pp. 295-314. In: Manual of Clinical Microbiology. 7th Edition. P. R. Murray (ed.). American Society for Microbiology, Washington, DC.
- 2008 draft. Bille, J. B. Catimel, E. Bannerman, C. Jacquet, M.N. Yersin, I. Camiaux, D. Monget and J. Rocourt. 1992. API Listeria, a new and promising one-day sysem to identify Listeria isolates. Appl. Environ. Microbiol. 58(6):1857-1860.
- Boerlin et al. 1992. L. ivanovii subsp. londoniensis subsp. novi. Int. J. Syst. Bacteriol. 42:69-73.
- Blodgett, R. 2006. Appendix 2. Most Probable Number from Serial Dilutions. In U.S. Food and Drug Administration Bacteriological Analytical Manual Online.
- Christie, R., N. E. Atkins, and E. Munch-Petersen. 1944. A note on the lytic phenomenon shown by group B streptococci. Aust. J. Exp. Biol. Med. Sci. 22: 197-200.
- Curiale, M. S., T. Sons, L. Fanning, W. Lepper & D. McIver. 1994. Deoxyribonucleic acid hybridization method for the detection of Listeria in dairy products, seafoods, and meats: collaborative study. J. AOAC INTERNATIONAL 77:602-617.
- Curiale, M. S., W. Lepper & B. Robison. 1994. Enzyme-linked immunoassay for detection of Listeria monocytogenes in dairy products, seafoods, and meats: collaborative study. J. AOAC INTERNATIONAL 77:1472-1489.
- Curtis, G. D. W., R. G. Mitchell, A. F. King, and J. Emma. 1989. A selective differential medium for the isolation of Listeria monocytogenes. Lett. Appl. Microbiol. 8:95-98.
- Feldsine, P. T., A. H. Lienau, R. L. Forgey, and R. D. Calhoon. 1997. Assurance polyclonal enzyme immunoassay (EIA) for detection of Listeria monocytogenes and related Listeria species in selected foods: collaborative study. J. AOAC INTERNATIONAL 80:775-790.
- Feldsine, P. T., A. H. Lienau, R. L. Forgey & R. G. Calhoon. 1997. Visual immunoprecipitate assay (VIP) for Listeria monocytogenes and related Listeria species detection in selected foods: collaborative study. J. AOAC INTERNATIONAL 80:791-805.
- Gangar, V., M. S. Curiale, A. D'Onorio, A. Schultz, R. L. Johnson, and V. Atrache. 2000. VIDAS® Enzyme-linked immunofluorescent assay for detection of Listeria in foods: collaborative study. J. AOAC INTERNATIONAL 83:903-918.
- Higgins, D. L., and B. J. Robison. 1993. Comparison of MICRO-ID Listeria method with conventional biochemical methods for identification of Listeria isolated from food and environmental samples: collaborative study. J. AOAC INTERNATIONAL 76:831-838.
- Hitchins, A. D. 1998. Listeria monocytogenes. Chapter 10. In: G. J. Jackson (Coordinator) Bacteriological Analytical Manual. 8th Edition. Revision A. AOAC INTERNATIONAL, Gaithersburg, MD.
- Hitchins, A. D., and R. E. Duvall. 2000. Feasibility of a defined microflora challenge method for evaluating the efficacy of foodborne Listeria monocytogenes selective enrichments. J. Food Protect. 63:1064-1070.
- Jinneman, K., J. M. Hunt, C. A. Eklund, J. S. Wernberg, P. N. Sado, J. M. Johnson, R. S. Richter, S. T. Torres, E. Ayotte, S. J. Eliasberg, P. Istafanos, D. Bass, N. Kexel-Calabresa, W. Lin,, and C. N. Barton. 2003. Evaluation and Interlaboratory Validation of a Selective Agar for Phosphatidylinositol-Specific Phospholipase C Activity Using Chromogenic Substrate to Detect Listeria monocytogenes from Foods. J. Food Protect. 66:441-445.
- Johnson, J.M., K. Jinneman, G. Stelma, B. G. Smith, D. Lye, J. Messer, J. Ulaszek, L. Evsen, S. Gendel, R. W. Bennett, B. Swaminathan, J. Pruckler, A. Steigerwalt, S. Kathariou, S. Yildirim, D. Volokhov, A. Rasooly, V. Chizhikov, M. Wiedmann, E. Fortes, R. E. Duvall, and A. D. Hitchins. 2004. Natural Atypical Listeria innocua Strains with Listeria monocytogenes Pathogenicity Island 1 Genes. Appl. Environ. Microbiol. 70:4256-4266.
- Jones, D., and H.P.R. Seeliger. 1986. International committee on systematic bacteriology. Subcommittee the taxonomy of Listeria. Int. J. Syst. Bacteriol. 36:117-118.
- Jones, D. 1992. Current classification of the genus Listeria. In: Listeria 1992. Abstracts of ISOPOL XI, Copenhagen, Denmark). p. 7-8.
- Knight, M. T., M. C. Newman, M. Joseph-Benziger Jr., J. R. Agin, M. Ash, P. Sims, and D. Hughes. 1996. TECRA Listeria Visual Immunoassay [TLVIA] for detection of Listeria in foods: collaborative study. J. AOAC INTERNATIONAL 79:1083-1094.
- Lee, W. H., and D. McClain. 1986. Improved L. monocytogenes selective agar. Appl. Environ. Microbiol. 52:1215-1217.
- Mattingly, J. A., B. T. Butman, M. C. Plank, and R. J. Durham. 1988. A rapid monoclonal antibody-based ELISA for the detection of Listeria in food products. J. AOAC INTERNATIONAL 71:669-673.
- Official Methods of Analysis of AOAC INTERNATIONAL AOAC INTERNATIONAL, Gaithersburg, MD, USA Official Method 2002.09
- Official Methods of Analysis of AOAC INTERNATIONAL AOAC INTERNATIONAL, Gaithersburg, MD, USA Official Method 2004.02
- Official Methods of Analysis of AOAC INTERNATIONAL AOAC INTERNATIONAL, Gaithersburg, MD, USA Official Method 2004.06
- Official Methods of Analysis of AOAC INTERNATIONAL AOAC INTERNATIONAL, Gaithersburg, MD, USA Official Method 2010.02
- Official Methods of Analysis of AOAC INTERNATIONAL AOAC INTERNATIONAL, Gaithersburg, MD, USA Official Method 2012.02
- Official Methods of Analysis of AOAC INTERNATIONAL AOAC INTERNATIONAL, Gaithersburg, MD, USA Official Method 2013.10
- Official Methods of Analysis of AOAC INTERNATIONAL AOAC INTERNATIONAL, Gaithersburg, MD, USA Official Method 2013.11
- Restaino, L., E. W. Frampton, R. M. Irbe, G. Schabert, and H. Spitz. 1999. Isolation and detection of Listeria monocytogenes using fluorogenic and chromogenic substrates for phosphatidylinositol-specific phospholipase C. J. Food Protect. 62:244-251.
- Rocourt, J., P. Boerlin, F.Grimont, C. Jacquet, and J-C. Piffaretti. 1992. Assignment of Listeria grayi and Listeria murrayi to a single species, Listeria grayi, with a revised description of Listeria grayi. Int. J. Syst. Bacteriol. 42:171-174.
- Rocourt, J. 1999. The genus Listeria and Listeria monocytogenes: phylogenetic position, taxonomy, and identification. In: Listeria, Listeriosis and Food Safety. E. T. Ryser and E. H. Marth (Eds). 2nd edition, pp. 1-20. Marcel Dekker, Inc., New York, NY.
- Ryser, E. T., and E. H. Marth. 1999. Listeria, Listeriosis and Food Safety. 2nd edition. Marcel Dekker, Inc., New York, NY.
- Seeliger, H.P.R., and D. Jones. 1986. Listeria. pp. 1235-1245. In: Bergey's Manual of Systematic Bacteriology, Vol. 2, 9th ed. P.H.A. Sneath, N.S. Mair, M.E. Sharpe, and J.G. Holt (Eds). Williams & Wilkins Co., Baltimore, MD.
- Shaw S., Nundy D. and Blais B.: Performance of the ALOA medium in the detection of hemolytic Listeria species in food and environmental samples. Laboratory Services Division, Canadian Food Inspection Agency, Ottawa, Ontario, Canada K1A 0C6.
- Stelma, G.N., Jr., A. L. Reyes, J. T. Peeler, D.W. Francis, J.M. Hunt, P L. Spaulding, C.H. Johnson, and J. Lovett. 1987. Pathogenicity testing for L. monocytogenes using immunocompromised mice. J. Clin. Microbiol. 25:2085-2089.
- USDA/FSIS. 1999. Isolation and identification of Listeria monocytogenes from red meat, poultry, egg and environmental samples. Ch. 8. Microbiology Laboratory Guidebook. 3rd Edition, Revision 2.
- US FDA/CFSAN. 2008. Guidance for Industry: Control of Listeria monocytogenes in Refrigerated or Frozen Ready-To-Eat Foods (Draft Guidance). (accessed 04/14/2011).
- US DHHS/FDA/CFSAN and USDA/FSIS. 2003. Listeria monocytogenes Risk Assessment: Quantitative Assessment of Relative Risk to Public Health from Foodborne Listeria monocytogenes among Selected Categories of Ready-to-Eat Foods. (accessed 04/14/2011).
- Vlaemynck G., Lafarge V., Scotter S. (2000): Improvement of the detection of Listeria monocytogenes by the application of ALOA, a diagnostic, chromogenic isolation medium. Journal of Applied Microbiology, 88 : 430-441.
- Van Netten et al. 1989. Liquid and solid selective differential media for the detection and enumeration of Listeria monocytogenes. Int. J. Food Microbiol. 8:299-316.
- Wang, S-Y. and A. D. Hitchins. 1994. Differential enrichment kinetics of severely and moderately injured Listeria monocytogenes cell fractions of heat injured populations. J. Food Safety 14:259-27.