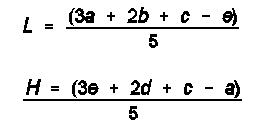BAM Chapter 20A: Inhibitory Substances in Milk
Bacteriological Analytical Manual (BAM) Main Page
January 2001
Author: Larry J. Maturin (ret.)
Two methods for detecting substances that inhibit microbes in milk are the cylinder plate method, using Micrococcus luteus as the test organism, and a paper disk method, using Bacillus stearothermophilus. The cylinder plate method, which is the official method for quantitative detection of β-lactam residues (3), is described in Chapter 16, Dairy Products, in Official Methods of Analysis (2). The analyst must also refer to Chapter 42, Drugs in Feeds (Official Methods), for preparation of media and certain other details. The entire method is presented here for convenience.
A description of the cylinder plate method for detecting penicillin in dry powdered milk is given by Kramer et al. (6). The same basic procedure can be applied to the assay of penicillin in fluid milk. Normal whole milk is used as a control diluent in place of the dry powdered milk plus buffer control diluent listed under the procedure for Preparation of Standard Curve. The normal whole milk that is to serve as the control diluent should be tested before use to ensure that it exhibits no antibacterial activity against the test organism. No sample preparation is necessary; milk samples submitted for examination are tested just as they are received. In all cases, the milk samples should be fresh. If a milk sample is suspected to contain penicillin at a level greater than 0.2 units/ml, it should be diluted with the control diluent to an estimated 0.05 unit/ml concentration before being tested. All other parts of the procedure remain the same. The disk method, which is the official method for qualitative detection of inhibitory substances in milk (1), is a modification of the method approved by the International Dairy Federation for the qualitative detection of penicillin in milk (4).
Micrococcus luteus Cylinder Plate Method
- Equipment and Materials
- Stainless steel cylinders, 8 + 0.1 mm od, 6 + 0.1 mm id, 10 + 0.1 mm long. Available from S & L Metal Products Corp., 58-29 57th Drive, Maspeth, NY 11378.
- Shaw cylinder dispenser. Available from E.C. Condit, P.0. Box 75, Middle Haddam, CT 06456, or Arthur E. Farmer, P.O. Box 1785, Trenton, NJ 08618.
- Petri dishes, 20 × 100 mm, with porcelain covers, glazed on outside, or cover lids with filter pads able to absorb water of syneresis. Comparable plastic petri dishes may be used if desired. Plastic covers may be used if they are raised slightly to let water escape.
- Media and Reagents
- Antibiotic Medium No. l (M14). Penassay seed agar (Difco) and seed agar (BBL) are satisfactory.
- Antibiotic Medium No. 4 (M15). Yeast beef agar (Difco and BBL) are satisfactory.
- Phosphate buffer, 1% (pH 6.0 + 0.1). Dissolve 8.0 g monobasic potassium phosphate and 2.0 g dibasic potassium phosphate in distilled water and dilute to 1 liter with distilled water.
- Penicillinase (β-lactamase) (R55). Available from Difco Laboratories, Box 1058A, Detroit, MI 48232; BBL, Div. of BioQuest, P.O. Box 243, Cockeysville, MD 21030; ICN Nutritional Biochemicals, 26201 Miles Road, Cleveland, OH 44128; Schwarz/Mann, Orangeburg, NY 10962; Calbiochem, 10933 N. Torrey Pines Road, La Jolla, CA 92037.
- Penicillin G working standard. Authentic penicillin G reference standard may be obtained from U.S.P. Reference Standards, 12601 Twinbrook Parkway, Rockville, MD 20852. Follow label directions for preparation and storage. Prepare stock solutions by carefully weighing, in an atmosphere of 50% humidity or less, a small amount of standard ana diluting weighed powder in appropriate diluent to obtain solution of convenient concentration.
- Physiological saline solution, 0.85% (sterile) (R63)
-
Preparation of Micrococcus luteus
Cultures of Micrococcus luteus (ATCC 9341) may be obtained from American Type Culture Collection, 10801 University Boulevard, Manassas, VA 20110-2209. Maintain as stock culture on agar slants of Antibiotic Medium No. 1 and transfer to fresh slant about once every 2 weeks. Prepare suspension as follows: Streak agar slant heavily with test organism and incubate 18-24 h at 32-35°C. Wash growth from slant with 1-2 ml sterile physiological saline and transfer to dry surface of Roux bottle containing 300 ml Antibiotic Medium No. 1. Spread suspension evenly over entire surface with aid of sterile glass beads. Incubate 18-24 h at 32-35°C. Wash growth from agar surface with 50 ml saline. Before actual assay, prepare trial plates to determine optimum amount of bulk suspension to be added to seed agar to obtain best zones of inhibition. Generally this will range only from 0.1 to 0.5 ml inoculum per 100 ml Antibiotic Medium No. 4. Store this stock suspension in refrigerator no longer than 2 weeks.
-
Preparation of plates
Add 10 ml Antibiotic Medium No. 1 to each petri dish. Distribute medium evenly and let harden on flat, level surface. Melt Antibiotic medium No. 4, cool to 48°C, and add optimum amount of culture per 100 ml, as determined above. Mix thoroughly. Add 4.0 ml of this inoculated agar to each plate. Distribute agar evenly by tilting plates from side to side in circular motion. Let harden. Use plates the same day they are prepared.
-
Preparation of standard curve
Prepare stock solution of penicillin G by dissolving accurately weighed portion of penicillin standard in enough phosphate buffer (B-3, above) to obtain solution containing 1000 units/ml. This stock solution may be used for 2 days. Prepare control diluent from phosphate buffer and antibiotic-free dry powdered milk at concentration of 3 ml buffer per 1.0 g milk. Further dilute penicillin G stock solution in control diluent to obtain concentrations of 0.00625, 0.0125, 0.025, 0.05, 0.1, and 0.2 unit/ml. Reference concentration is 0.05 unit/ml. Place 6 cylinders, equally spaced, on inoculated agar surface of prepared petri plates (D, above). Fill 3 cylinders with 0.05 unit/ml standard solution and 3 cylinders with one other concentration of standard, alternating so that each 0.05 unit/ml cylinder is followed by cylinder of the other concentration. Use 3 plates for each point in curve, for total of 15 plates. The 3 plates containing lowest concentration of standard (0.00625 unit/ml) are intended to produce negative results; other 12 plates are used to construct standard response line. This gives 45 determinations for 0.05 unit/ml and 9 determinations for each of other points on the line.
Replace covers and incubate plates 16-18 h at 30°C. After incubation, invert plates to remove cylinders. Measure diameter of each zone of inhibition as accurately as possible (at least to nearest 0.5 mm). Average readings of 0.05 unit/ml concentration and of point tested on each set of 3 plates. Also average all 45 readings of 0.05 unit/ml concentration to obtain correction point for curve.
Correct average value for each point to value it would be if average of 0.05 unit/ml readings for that set of 3 plates were same as correction point. Example: If average of 45 readings of 0.05 unit/ml concentration is 20 mm, and average of nine 0.05 unit/ml cylinders on set of three 0.025 unit/ml plates is 19.8 mm, correction is + 0.2 mm. If average reading for nine 0.025 unit/ml cylinders on these 3 plates is 17.0 mm, corrected value is 17.2 mm. Plot corrected values, including average of 0.05 unit/ml concentration, on 2-cycle, semilog graph paper, placing concentration in units/ml on logarithmic scale and diameter of zone of inhibition on arithmetic scale. Construct best straight line through these points, either by inspection or by using following equations:
where L and H = calculated zone diameters for lowest and highest (0.0125 and 0.2 unit/ml) concentrations of standard response line; c = average zone diameter of 45 zone diameters for reference concentration; and a, b, d, e = corrected average zone diameters for each of other concentrations used for standard response line.
-
Preparation of sample
Accurately weigh 10 g dry powdered milk sample and add 30 ml phosphate buffer (B-3, above). Mix thoroughly. If concentration of penicillin G greater than 0.2 unit/ml in this mixture is expected, dilute aliquot of reconstituted powdered milk with control diluent (E, above) to estimate 0.05 unit/ml. To identify activity as penicillin, take portion of sample, add penicillinase concentrate (B-4, above) at rate of 0.5 ml/10 ml sample, and incubate 30 min at 37°C. On 3 plates, fill 2 cylinders with unit/ml reference standard. 2 cylinders with untreated sample, and 2 cylinders with penicillinase-treated sample. Incubate plates and follow same procedure as in E, above. Zone of inhibition with untreated sample and no zone with penicillinase-treated sample is positive test for penicillin G.
-
Calculation of potency
Average the zone readings of standard and zone readings of sample on 3 plates. If average zone size of sample is larger than average for standard, add difference between them to zone size of reference standard on curve. If average sample value is lower than standard value, subtract difference between them from zone size of reference standard on curve. From curve, read concentration corresponding to this adjusted sample zone size. Multiply concentration in units/ml by dilution factor of 4X to obtain final concentration of penicillin G in units/g. If sample powder has been additionally diluted, appropriate dilution factor must be taken into account in calculation of final potency.
-
Controls
In using these assay procedures, the analyst must be certain that any antibiotic activity detected derives from the sample and not from environmental conditions (including the analyst), the equipment, or reagents used. Good laboratory practices require that proper controls be maintained throughout the analytical process. These should always include controls to indicate the degree of precision and accuracy of the determinations to be reported. The lowest standard concentration (0.00625 unit/ml) is intended to be a control that may produce negative results. This lowest concentration represents control diluent to which the drug has been added at a level that is normally below the limit of detectability. On occasion, the lowest concentration (0.00625 unit/ml) will produce a measurable zone of inhibition. The next highest concentration (0.0125 unit/ml) should always produce positive results. The sensitivity of this assay is normally 0.01 unit/ml. The control diluent should always produce negative results.
Bacillus stearothermophilus Disk Assay - Qualitative Method II
- Equipment and Materials
- PM indicator agar (M6)
- Trypticase (tryptic) soy broth without dextrose (M154)
- Trypticase (tryptic) soy agar (M152)
- Petri dishes (see A-3, above)
- Paper disks, 12.7 mm (Schleicher & Schuell)
- Penicillinase (β-lactamase) (R55)
- Control disks. Prepare fresh daily from positive control milk containing 0.008 unit/ml penicillin.
- 90 µl pipettor with disposable tips
- Vernier calipers
- Fine tip forceps
-
Penicillin G working standard
Accurately weigh, in an atmosphere of less than 50% relative humidity, about 30 mg U.S.P. sodium penicillin G reference standard (U.S.P.). Dissolve in enough phosphate buffer to obtain stock concentration of 100-1000 units/ml. Store in dark for <2 days="" at="">
-
Preparation of Bacillus stearothermophilus
Cultures of B. stearothermophilus var. calidolactis (ATCC 10149) (5) may be obtained from American Type Culture Collection, 10801 University Boulevard, Manassas, VA 20110-2209. Maintain on trypticase soy agar slants and transfer weekly. Prepare spore suspension as follows: Inoculate three 300 ml Erlenmeyer flasks, each containing 150 ml trypticase soy broth without dextrose. Incubate flasks at 64 ± 2°C. Periodically make spore stains to determine extent of sporulation. When sufficient sporulation has occurred (about 80%, usually in 72 h), centrifuge cells 15 min at 5000 rpm. Decant supernatant, resuspend cells in physiological saline, and recentrifuge; repeat. After discarding supernatant from final wash, suspend cells in 30 ml physiological saline and store at 0-4.4°C. Spore suspension will remain viable 6-8 months. Commercially prepared spore suspension is satisfactory. Check viability periodically by preparation of trial test plates.
-
Preparation of standard milk solution
Prepare standard milk solution by diluting penicillin G stock solution in inhibitor-free milk to concentration of 0.008 unit/ml. Store at 0-4.4°C for not more than 2 days, or distribute in small amounts and freeze in non-frost-free freezer. Store frozen no longer than 6 months. Difco PM positive controls and Penicillin Assay, Inc., penicillin standards are satisfactory.
-
Preparation of plates
Inoculate PM indicator agar, cooled to 64°C, with previously prepared spore suspension of B. stearothermophilus (C, above). Adjust inoculum level to provide 1 × 106 spores/ml. Pipet 6 ml inoculated agar into each petri dish and let harden on level surface. Use plates fresh or store in sealed plastic sacks at 0-4.4°C and use within 5 days of preparation.
-
Assay - Screening
Use 90 µl pipettor. With tip securely fastened and pipettor positioned vertically, depress plunger completely to first stop. Insert tip 1 cm below surface of well-mixed sample (if necessary, tilt sample to avoid foam). Release plunger slowly to fill tip properly. (If plunger is released too quickly, amount of milk taken up will not be uniform. If tip is not appropriately filled after releasing plunger, repeat above procedure.) With clean, dry forceps, remove and place a blank disk (within identified section) on agar surface, pressing disk gently with forceps to ensure good contact. Immediately deliver sample to disk. Holding pipettor vertically with tip approximately 1 cm above center of disk, depress plunger in slow, continuous motion to first stop. With plunger completely depressed (go to the second stop, if one exists) touch off tip once to center of disk. Observe that tip is empty before discarding. (Repeat above for all samples.) Prepare control disk, containing 0.008 unit penicillin/ml, as for above samples.
Alternatively, with clean, dry forceps, touch paper disk to surface of well-mixed milk. (Agitate raw samples by shaking 25 times in 7 s through arc of 1 ft or completely invert retail containers 25 times. Let bubbles break up before sampling. Sample must be taken within 3 min of agitation.) Let milk be absorbed by capillary action. Drain excess milk by touching edge of disk once to inside surface of sterile petri dish lid. Immediately place disk on agar surface, pressing gently to ensure good contact. (Repeat above for all samples.) Place control disk containing 0.008 unit penicillin/ml on agar surface as above (if multiple plates, vary location of control on each plate, center or edge). Identify each disk or section on which it is placed. (Place a maximum of 7 disks/plate, 6 edge and 1 center.)
Before inverting plate(s) check to see that disks are completely and uniformly filled by observing through bottom of petri dish at light source. Also determine that no milk is visible beyond edge of disks. Invert plates and incubate at 64 ± 2°C until well-defined zones of inhibition (16-20 mm) are obtained with 0.008 unit/ml control (2.5-3.5 h). Examine plate(s) for clear zone(s) of inhibition surrounding disks. (Measure zone(s) with Vernier calipers.) Clear zones of 14 mm indicate presence of inhibitory substances. Zones of <14 mm are read as negative. Zones of 16 mm must be confirmed for the presence of inhibitor.
-
Assay - Confirmatory
Heat test sample to 82°C for >2 min and cool promptly to room temperature. Use 90 µl pipettor procedure or, alternatively, with clean, dry forceps, touch paper disk to surface of well-mixed milk and let milk be absorbed by capillary action. Add 0.05 ml penicillinase to 5 ml sample and fill disk. Drain excess milk by touching disk to inside surface of sterile petri dish. Immediately place each disk on agar surface, pressing gently to ensure good contact. Place control disk containing 0.008 unit/ml on plate, or use 90 µl pipettor (F, above). Invert plate and incubate at 64 ± 2°C until well-defined zones of inhibition (16-20 mm) are obtained with the 0.008 unit/ml control. Examine plate for clear zone of inhibition (>16 mm) surrounding disk, indicating presence of inhibitory substance.
- Interpretation - Assay of test milk in screening and confirmatory test may produce the following results:
- No zone around disk containing untreated milk in screening test is a negative test for inhibitory substances.
- Zone around disk containing untreated milk but no zone around disk containing penicillinase-treated milk in the confirmatory test is a positive test for β-lactam residue.
- Clear zone of equal size around both disks in confirmatory test indicates presence of inhibitors other than β-lactam residues.
- Clear zone of 4 mm around penicillinase-treated milk smaller than that around untreated milk disk in confirmatory test indicates presence of -lactam residues as well as another inhibitor(s).
The penicillin-positive control solution at 0.008 unit/ml should produce clear, well-defined zones of inhibition (16-20 mm). If no zone of inhibition is produced by penicillin-positive control, test sensitivity is not adequate and test should be repeated.
References
- Association of Official Analytical Chemists. 1982. Changes in methods. J. Assoc. Off. Anal. Chem. 65:466-467.
- Association of Official Analytical Chemists. 1984. Official Methods of Analysis, 14th ed., secs 16.163; 42.299-42.303. AOAC, Arlington, VA.
- Code of Federal Regulations. 1976. Title 21, sec. 436.105, U.S. Government Printing Office, Washington, DC.
- International Dairy Federation. 1970. Detection of penicillin in milk by a disk assay technique. International Dairy Federation, Brussels, Belgium.
- Kabay, A. 1971. Rapid quantitative microbiological assay of antibiotics and chemical preservatives of a nonantibiotic nature. Appl. Microbiol. 22:752-755.
- Kramer, J., G.G. Carter, B. Arret, J. Wilner, W.W. Wright, and A. Kirshbaum. 1968. Antibiotic residues in milk, dairy products and animal tissues: methods, reports and protocols. Food and Drug Administration, Washington, DC.
Hypertext Source: Inhibitory Substances in Milk, Bacteriological Analytical Manual, 8th Edition, Revision A, 1998. Chapter 20A.