ESG Appendix J: AS2 Routing IDs
ESG User Guide - Table of Contents
As an alternative to using the AS2 Header Attributes (Appendix G) process for sending submissions to the FDA ESG, you can use unique routing IDs for your submissions to indicate the type of submission and the intended Center. This Appendix describes how to automate the selection of the routing ID in Cyclone/Axway products through the incoming back-end integration pickup. If you are not using a Cyclone/Axway product, refer to your gateway software's documentation for help configuring routing IDs.
The routing IDs for each submission type are listed at the end of the appendix.
Before routing IDs can be selected, they must be added to your partner profile.
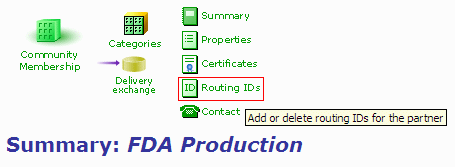
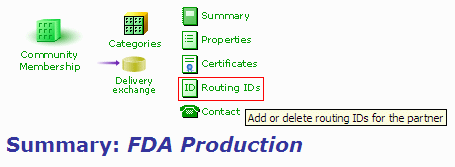
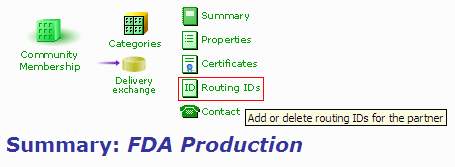
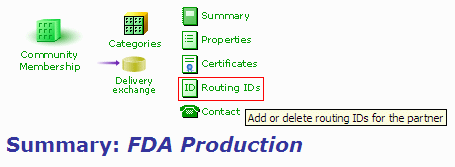
- In your FDA partner profile, click on the "Routing IDs" link.
Figure 1: Partner Profile
- Use the "Add" button at the bottom of the page to add extra routing IDs to the FDA partner.
Note: "ZZFDA" (production) or "ZZFDATST" (test) must be the first routing ID listed. Otherwise, the submission will not be processed correctly by the FDA.
Figure 2: Change Routing IDs Pages
Only file system integration pickups have the ability to automatically select a routing ID. If you are not currently using file system integration pickups to interface with the back-end server, you must create a file system integration pickup must be created. Refer to your gateway software's documentation for more information on this procedure.
- After you create a file system integration pickup, create a directory structure on the disk that matches the routing ID's path and configure the integration pickup to automatically select a routing ID based on directory names.
- In this example, the path to the integration pickup is C:\bin\cyclone\common\data\out, as shown in Figure 3: File System Settings, Directory Name. This path should match the directory structure you created in step 3.
Figure 3: File System Settings, Directory Name
Click on the Message attributes tab. This will access the following screen:
Figure 4: Message Attributes, Adding
Your list of Available attributes may differ from those shown. The only attribute here that must be on your list is To routing ID which is highlighted here.
- Click on To routing ID in the list of attributes. Then click on the Add button. After the screen refreshes, To routing ID should be listed under Selected attributes:
Figure 5: Message Attributes, Added
- Click on the Save changes button to commit the changes to the integration pickup.
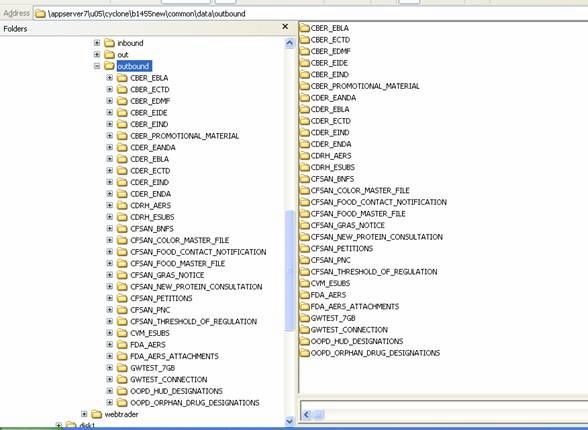
- Next, create the appropriate directories in the file system to match the FDA Routing IDs to which submissions are to be sent.
Figure 6: Available Routing shows a sample directory and subdirectories that correspond to some of the available routing IDs. A complete list of routing IDs for the FDA ESG are in Figure 7: Submission Types Supported by the FDA ESG.
- Create subdirectories beneath the base integration pickup directory as shown, then drop the documents that you wish to submit into the appropriate directory. For example, to send an AERS report, place the document in the "FDA_AERS" directory.
- For multi-document submissions, the original directory structure must be converted to a single file by using "tar" and then "gzip", and the resulting file must have an extension of .tar.gz for the FDA ESG to correctly recognize it as a multi-document submission. You must perform the tar and gzip procedure before dropping the file into the appropriate directory, since the Cyclone/Axway software does not know how to tar and gzip on its own.
Note: FDA_AERS and FDA_AERS_ATTACHMENTS submissions cannot be sent as multi-document submissions under any circumstances.
Figure 7: Submission Types Supported by the FDA ESG
Center Destination Submission Types Routing ID Test Production CBER
510K CBER_510K Yes Yes AERS FDA_AERS Yes Yes AERS_Attachments FDA_AERS_ATTACHMENTS Yes Yes BEST CBER_BEST Yes Yes CDISC CBER_CDISC Yes Yes EBLA CBER_EBLA Yes Yes EIDE CBER_EIDE Yes Yes EIND CBER_EIND Yes Yes eDMF CBER_EDMF Yes Yes EUA CBER_EUA Yes Yes H1N1_Lot_Release CBER_H1N1_LOT_RELEASE Yes Yes Lot_Release_Protocol CBER_ LOT_RELEASE_PROTOCOL Yes Yes NDA CBER_NDA Yes Yes PMA CBER_PMA Yes Yes PRE_IND CBER_PRE_IND Yes Yes Promotional_Material CBER_PROMOTIONAL_MATERIAL Yes Yes QSUBS CBER_QSUBS Yes Yes SEND_PILOT CBER_SEND_PILOT Yes No SPL_LDD CBER_SPLLDD Yes Yes VAERS CBER_VAERS Yes Yes CDER
ACA6004_Drug_Samples CDER_ACA6004 Yes Yes AERS FDA_AERS Yes Yes AERS_Attachments FDA_AERS_ATTACHMENTS Yes Yes AERS IND AERS_IND Yes No AERS Attachment IND AERS_ATTACHMENTS_IND Yes No AERS_PREMKT FDA_AERS_ PREMKT Yes No AERS_Attachments_PREMKT FDA_AERS_ATTACHMENTS_ PREMKT Yes No ECTD CDER_ECTD Yes Yes EIND CDER_EIND Yes Yes FFU-PILOT CDER_FFU-PILOT Yes No GDUFA_Facility_Registration CDER_GDUFA_FACILITY_REGISTRATION Yes Yes PFC CDER_PFC Yes Yes Voluntary_Direct_AEs WebTrader Submission ONLY N/A N/A CDRH
Adverse_Events CDRH_AERS Yes Yes Electronic_Submissions CDRH_ESUBS Yes Yes GUDID CDRH_GUDID Yes Yes CFSAN
Form 3480 CFSAN_FORM3480 Yes Yes Form 3480A CFSAN_FORM3480A Yes Yes Form 3666 CFSAN_FORM3666 Yes Yes Threshold_of_Regulation CFSAN_THRESHOLD_OF_REGULATION Yes Yes Form 3667 CFSAN_FORM3667 Yes Yes Form 3665 CFSAN_FORM3665 Yes Yes Form 3503 CFSAN_FORM3503 Yes Yes CTP
Electronic_Submission CTP_ESUB Yes Yes Adverse_Events CTP_AE No No CVM
Electronic_Submissions CVM_ESUBS Yes Yes Adverse_Events_Reports CVM_AERS Yes Yes eSubmitter CVM_ESUBMITTER Yes Yes CVM-VDD eSubmitter
(WebTrader: Electronic_Submissions)CVMVDD_eSubmitter Yes Yes OC SPL OC_REGLIST_SPL Yes Yes OOPD
HUD_Designation_Requests OOPD_HUD_DESIGNATIONS Yes No Orphan_drug_Designation_Requests OOPD_ORPHAN_DRUG_DESIGNATIONS Yes No ORA Inspection ORA_Inspection Yes No GWTEST
ConnectTest GWTEST_CONNECTION Yes No SizeTest GWTEST_7GB Yes No HC
Transaction HC_Transaction Yes Yes
- For multi-document submissions, the original directory structure must be converted to a single file by using "tar" and then "gzip", and the resulting file must have an extension of .tar.gz for the FDA ESG to correctly recognize it as a multi-document submission. You must perform the tar and gzip procedure before dropping the file into the appropriate directory, since the Cyclone/Axway software does not know how to tar and gzip on its own.
- In order to communicate the filename to the FDA, the FDA requires the FileName Preserve on all AS2 submissions.