Medical Device Common Entry Errors
Introduction
The Center for Devices and Radiological Health (CDRH) is the FDA center responsible for overseeing the medical device program. Medical devices range from simple tongue depressors and bedpans to complex programmable pacemakers with micro-chip technology and laser surgical devices.
The FDA defines a medical device as:
"an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part or accessory which is:
- Recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them,
- Intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or
- Intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes."
To expedite entry screening of medical device products by the Predictive Risk-based Evaluation for Dynamic Import Compliance Targeting (PREDICT) system, importers and entry filers must provide accurate product codes, all relevant affirmations of compliance (AofC) and accurate identifiers for firms, in addition to the general entry data.
When applicable entry data is supplied electronically, completely and accurately, it can be used by the PREDICT system to “look-up” the information in the FDA’s databases, validate the information, and issue a system May Proceed if the line is not held due to any other screening criteria.
When this additional entry information is not provided, or if it is provided incorrectly, the automated look-up will fail and the entry line may be subject to delays in processing.
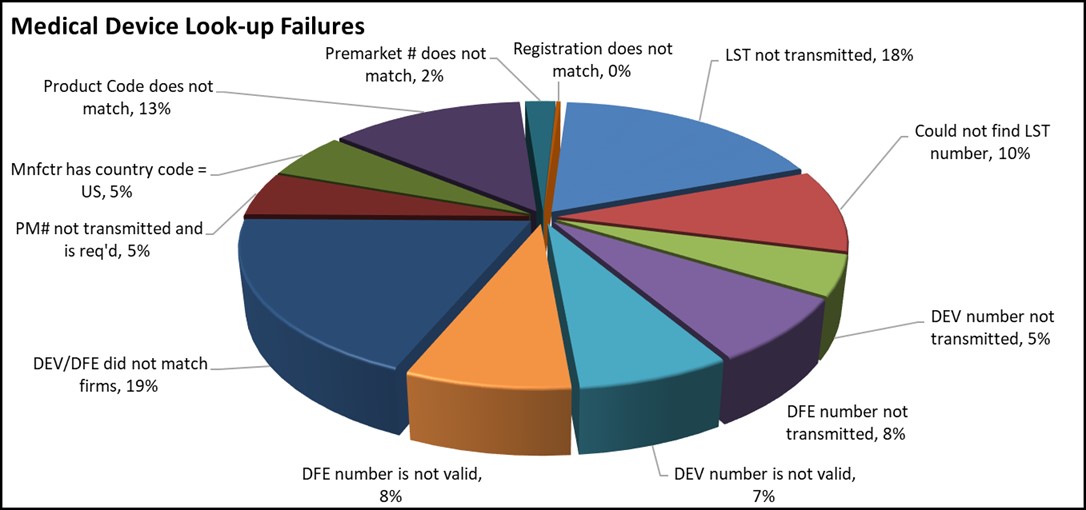
The following chart shows the most common look-up errors for medical device entries from 7/1/2021 - 9/30/2021.
The table below provides additional information regarding these common errors to help you provide accurate and complete information to the FDA.
Common Medical Device Look-up errors that result in a failed Look-up
|
Error |
Description |
Additional Information |
|---|---|---|
|
LST not transmitted |
No listing number (LST) was transmitted for the entry. Verify that a LST is required and provide one as applicable. There is no public website to search LSTs because they are proprietary.
|
LST from Manufacturer or Device Foreign Exporter are acceptable. To obtain a LST, search the public registration and listing database to obtain the contact information for the official correspondent. Contact the official correspondent of the firm that is required to list to request the LST. For more information see Who Must Register, List and Pay Fee |
|
Could not find LST number |
The LST transmitted could not be found in FDA's internal database. Verify that the LST provided is accurate and in the correct format. |
For help on LST definition and format, see the Affirmation of Compliance Code guides. The FDA cannot confirm, verify or provide LSTs to individuals that are not listed as the official correspondent on the firm’s registration. To confirm the LST is accurate contact the official correspondent of the firm that is required to list. For more information see Who Must Register, List and Pay Fee. |
|
DEV number not transmitted |
No registration number was transmitted for the foreign manufacturer (DEV). Verify that a DEV is required and provide one as applicable. If the firm has registered and the registration number has not yet been assigned, the owner/operator number may be provided until the registration number is assigned. |
For more information see Who Must Register, List and Pay Fee. To search for registration information see the Search Registration and Listing Database For US goods returned, Import for Export (IFE), reprocessing, and medical device kits, a domestic device manufacturer (DDM) may be required if DEV is not applicable. |
|
DFE number not transmitted |
No registration number was transmitted for the foreign device exporter (DFE). Verify that a DFE is required and provide one as applicable. If the firm has registered and the registration number has not yet been assigned, the owner/operator number may be provided until the registration number is assigned. |
For more information see Who Must Register, List and Pay Fee. To search for registration information see the Search Registration and Listing Database |
|
DEV number is not valid |
The DEV transmitted does not match a valid non-US firm in the FDA's internal database. Verify the DEV provided is accurate. |
For more information see Who Must Register, List and Pay Fee. To search for registration information see the Search Registration and Listing Database |
|
DFE number is not valid |
The DFE transmitted does not match a valid non-US firm in the FDA's internal database. Verify the DFE provided is accurate. |
For more information see Who Must Register, List and Pay Fee. To search for registration information see the Search Registration and Listing Database |
|
DEV/DFE did not match firms |
The DEV and/or DFE transmitted on the line did not match the firms transmitted. Verify that the registration information (DEV and/or DFE) transmitted match the manufacturer and shipper transmitted on the line.
|
When supplying manufacturer information through the Automated Commercial Environment (ACE), only firm name and address are required to be submitted; however, if a FEI number is also provided, that can improve the chances that firm name and address matches the registration number provided.
To find the FEI for a registered firm, search the firm’s registration number on the Search Registration and Listing Database; the FEI Number is located on the registration record. |
|
PM# not transmitted and is req'd |
Based on the product code provided, a PMN/PMA/PM# is required but none were transmitted on the line. Verify that the product code provided is accurate and provide a PMN/PMA/PM# Number as applicable. |
Certain medical devices require premarket submissions depending on use and classification. For additional information visit the Medical Device Overview webpage. To determine the submission type needed for a product code, search the three alpha characters of a device product code (for example, for product code 87L—BC use LBC) on the Search Product Classification Database; the submission type is displayed. |
|
Mnfctr has Country code = US |
The LST transmitted indicated that the manufacturer was a US firm, but the manufacturer provided on the line is a foreign firm. Verify the correct LST was provided for the foreign firm. |
The FDA cannot confirm, verify or provide LSTs to individuals that are not listed as the official correspondent on the firm’s registration. To confirm the LST is accurate contact the official correspondent of the firm that is required to list. |
|
Product Code does not match |
The product code transmitted for the entry did not match the product code on the LST transmitted. Verify that the correct LST was provided and/or that the product code matches the LST. |
To help determine the appropriate product code, visit the Product Classification searchable database webpage.
For additional assistance with building a product code visit FDA’s Product Code Builder webpage.
The FDA cannot confirm, verify or provide LSTs to individuals that are not listed as the official correspondent on the firm’s registration. To confirm the LST is accurate contact the official correspondent of the firm that is required to list.
|
|
Premarket # does not match |
The Premarket notification or Premarket approval (PM#) transmitted for the entry did not match the PM# number on the LST transmitted. Verify that the PM# provided matches the product being introduced for import. |
Certain medical devices require premarket submissions depending on use and classification. For additional information visit the Medical Device Overview webpage. To search for premarket submissions, visit the Medical Devices Databases webpage. |
|
Registration does not match |
The registration number provided in the entry did not match the registration number on the transmitted LST. Verify the DEV provided matches the LST provided. |
The FDA cannot confirm, verify or provide LSTs to individuals that are not listed as the official correspondent on the firm’s registration. To confirm the LST matches the DEV contact the official correspondent of the firm that is required to list.
|
|
UNK is declared as IUC and the AofC REG supplied instead of DEV |
When the Intended Use Code (IUC) is declared as UNK, inappropriate AofC qualifiers can be transmitted causing a lookup failure. Example: The Affirmation of Compliance (AofC) provided was for Drug Registration (REG) not Device Manufacturer Registration (DEV) or Device Foreign Exporter Registration (DFE) as required for a device entry. Make sure when submitting a foreign device registration number that either DEV (manufacturer) or DFE (exporter) is used. |
|