Center for Devices and Radiological Health's Response to Coronavirus (COVID-19): Infographic
"If you want to know the U.S. Food and Drug Administration's impact on COVID-19, look no further than the numbers." Stephen M. Hahn, M.D., Commissioner of Food and Drugs
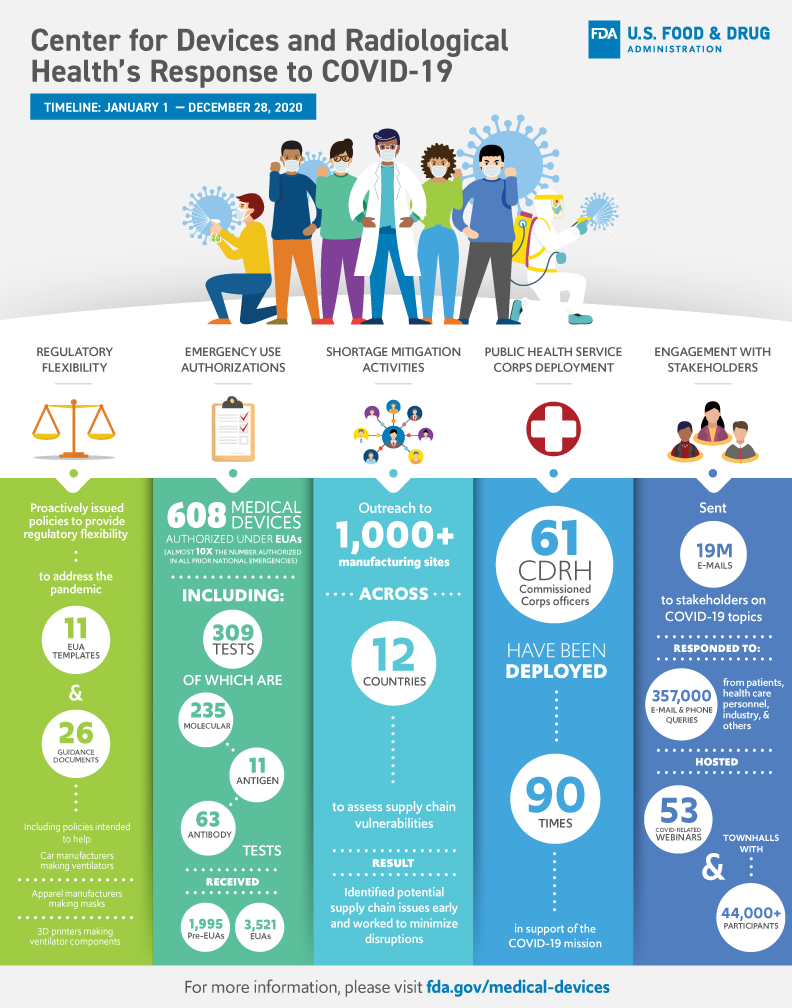
This infographic provides a visualization of data associated with CDRH's response to the coronavirus (COVID-19) pandemic in 2020.
Text Version of Infographic
Center for Devices and Radiological Health's Response to COVID-19
Timeline: January 1 - December 28, 2020
Regulatory Flexibility: Proactively issued policies to provide regulatory flexibility to address the pandemic
- 11 EUA templates
- 26 guidance documents
- Including policies intended to help:
- Car manufacturers making ventilators
- Apparel manufacturers making masks
- 3D printing making ventilator components
Emergency Use Authorizations: 608 medical products authorized under EUAs (almost 10x the number authorized in all prior national emergencies), including 309 tests and collection kits, of which:
- 235 are molecular tests and collection kits
- 11 are antigen tests
- 63 are antibody tests
The FDA received
- 1,995 pre-EUAs
- 3,521 EUAs
Shortage Mitigation Activities: Outreach to 1,000+ manufacturing sites across 12 countries to access supply chain vulnerabilities. Result: Identified potential supply chain issues early and worked to minimize disruptions
Public Health Service Corps Deployment: 61 CDRH Commissioned Corps officers have been deployed 90 times in support of the COVID-19 mission.
Engagement with Stakeholders:
- Sent 19 million emails to stakeholders on COVID-19 topics
- Responded to 357,000 email and phone queries from patients, health care personnel, industry, and others
- Hosted 53 COVID-related webinars and townhalls with 44,000+ participants
For more information, please visit fda.gov/medical-devices.