Manufacturing and Distributing Respirators for Health Care Personnel Use in the United States Under an Existing Emergency Use Authorization (EUA) During the COVID-19 Pandemic
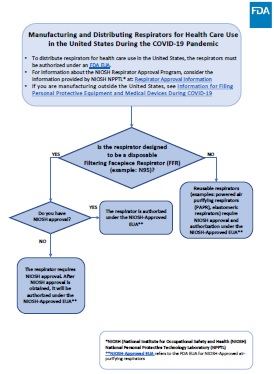
This flowchart provides information on manufacturing and distributing respirators for health care personnel use in the United States under an existing emergency use authorization (EUA) during the COVID-19 public health emergency. It also provides links to detailed information provided by the FDA and the Centers for Disease Control and Prevention (CDC)'s National Institute for Occupational Safety and Health (NIOSH).
Is the respirator designed to be a disposable Filtering Facepiece Respirator (FFR) (such as an N95)?
- NO: The product is a reusable respirator such as an elastomeric or powered air purifying respirator (PAPR). The emergency use of these respirators is authorized by the FDA if the device is NIOSH approved as set forth in the EUA for NIOSH-approved air-purifying respirators.
- YES: Continue to the following question:
Do you have NIOSH approval?
- YES: The respirator is authorized under the EUA for NIOSH-approved air-purifying respirators and can be distributed for health care personnel use in the United States during the COVID-19 pandemic. All FDA approved or cleared respirators are NIOSH-approved. Respirators approved by NIOSH are shown in the NIOSH Certified Equipment Lists (CEL) for non-powered air purifying respirators with particulate protection and PAPRs with particulate protection.
- NO: The respirator requires NIOSH approval (which may lead to FDA authorization as set forth in the EUA for NIOSH-approved air-purifying respirators) or FDA clearance.
For information on importing products, refer to Information for Filing Personal Protective Equipment and Medical Devices during COVID-19.
Note: For information about the types of filtering facepiece respirators (FFRs) for health care use, including disposable N95s, reusable elastomeric respirators, and reusable powered air purifying respirators (PAPRs) see the FDA's N95 Respirators, Surgical Masks, and Face Masks and the CDC's Respirator Trusted-Source Information.