About the Digital Health Center of Excellence
The Digital Health Center of Excellence (DHCoE) is part of the planned evolution of the Digital Health Program in the Center for Devices and Radiological Health (CDRH) and will align and coordinate digital health work across the FDA. It marks the beginning of a comprehensive approach to digital health technology, setting the stage for advancing and realizing the potential of digital health.
The DHCoE provides regulatory advice and support to the FDA’s regulatory review of digital health technology and is not responsible for making marketing authorization decisions.
On this page:
- Who We Are
- What We Do
- Who We Serve
- The Evolution of the Center of Excellence in 2020 and 2021
- The FDA’s Plan for Advancing Digital Health
- Frequently Asked Questions
Who We Are
We are a pool of digital health resources across the Agency who are aligned and working towards the goals of the Digital Health Center of Excellence. The DHCoE is part of the U.S. Food and Drug Administration (FDA), based in the Center for Devices and Radiological Health (CDRH).
What We Do
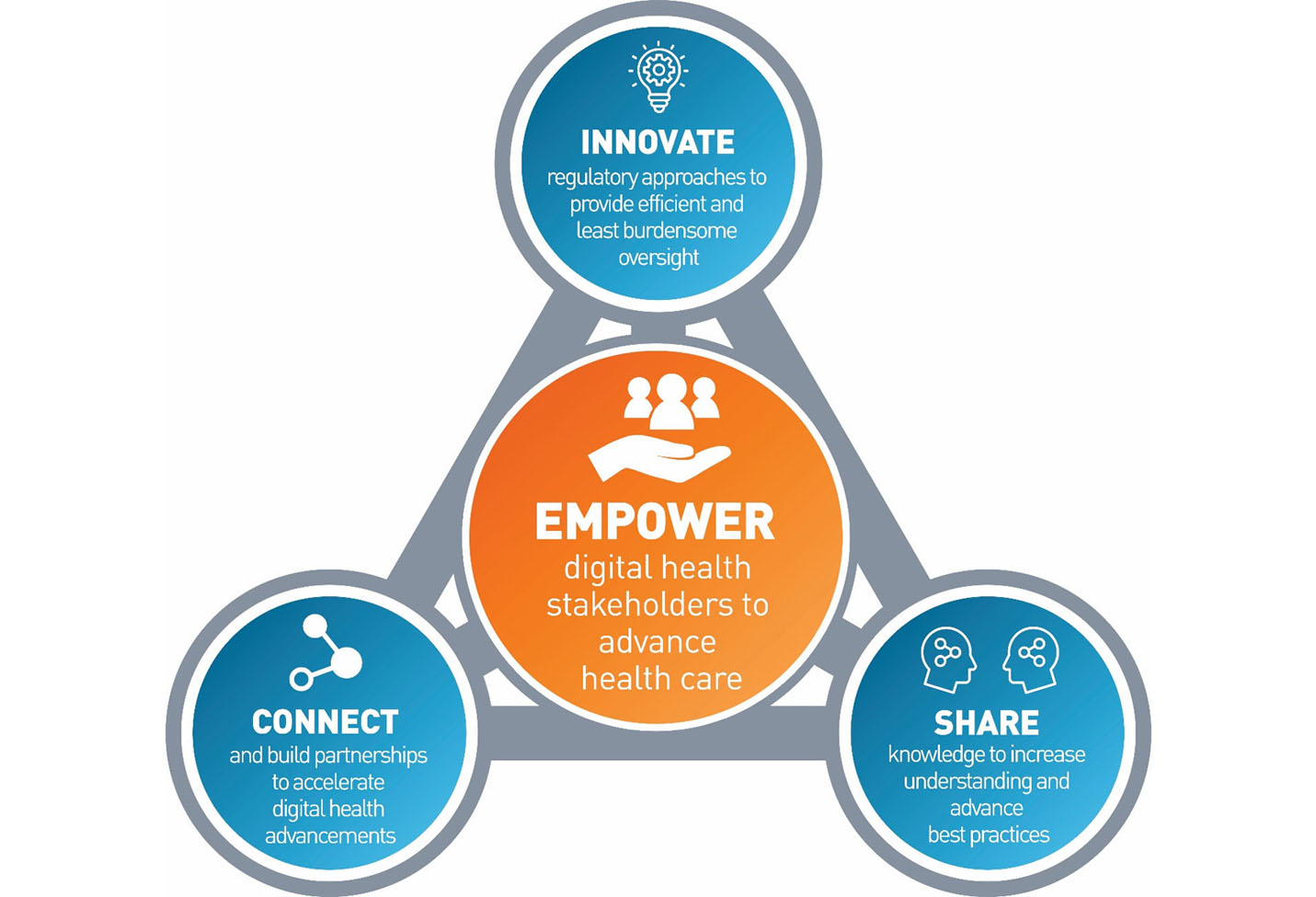
The Digital Health Center of Excellence empowers digital health stakeholders to advance health care by fostering responsible and high-quality digital health innovation. We provide services in the following functional areas for digital health:
- Digital Health Policy and Technology Support and Training
- Medical Device Cybersecurity
- Artificial Intelligence / Machine Learning
- Regulatory Science Advancement
- Regulatory Review Support and Coordination
- Advanced Manufacturing
- Real World Evidence and Advanced Clinical Studies
- Regulatory Innovation
- Strategic Partnerships
For details about the services that we provide, see Digital Health Services.
Who We Serve
Our customers include patients, developers, health care providers, researchers, industry, payers, other government agencies, international regulatory bodies, CDRH, and other centers within the FDA.
The Evolution of the Center of Excellence in 2020 and 2021
The evolution of the Digital Health Center of Excellence will occur in multiple phases to meet the goal of realizing the full potential of digital health.
|
Phases |
Focus |
Activities |
|---|---|---|
|
Phase 1 |
Raise Awareness and Engage Stakeholders |
|
|
Phase 2 |
Build Partnerships |
|
|
Phase 3 |
Build and Sustain Capacity |
|
The FDA’s Plan for Advancing Digital Health
The Digital Health Innovation Action Plan outlines our efforts to reimagine the FDA's approach to ensuring all Americans have timely access to high-quality, safe, and effective digital health products. As part of this plan, we committed to several key goals:
- Issuing guidance to modernize our policies.
- Increasing the number and expertise of digital health staff at the FDA.
- Developing the Digital Health Software Precertification Pilot Program ("Pre-Cert")
Frequently Asked Questions About the Digital Health Center of Excellence
Q: What is digital health?
A: Read the FDA definition of digital health and its benefits.
Q: Why was the Digital Health Center of Excellence created?
A: The Digital Health Center of Excellence (DHCoE) was created to empower stakeholders to advance health care by fostering responsible and high-quality digital health innovation. The DHCoE is part of the planned evolution of the Digital Health Program in CDRH. Its creation marks the beginning of a comprehensive approach to digital health technology, to advance and realize its full potential.
Q: Does the DHCoE review submissions for digital health technology?
A: While the DHCoE supports the FDA’s review and decision-making process for digital health technology, it is not responsible for making marketing authorization decisions. For more information on the process for marketing authorizations please follow the FDA’s current review processes.
Q: What changes with the launch of the DHCoE?
A: The DHCoE sets a stage to advance digital health technologies and creates a platform to bring all digital health efforts to be aligned towards one goal. FDA review teams will continue to leverage the FDA’s capacity and expertise in digital health. The DHCoE will help set digital health regulatory science research priorities for CDRH, and coordinate research in mutual areas of interest for other FDA centers. The DHCoE will also work towards developing a knowledge hub of tools and information for policy development and implementation, and a one-stop-shop for digital health related inquiries as well as partnerships and collaborations.
Q: I’m a software developer or new innovator. How will the DHCoE help me?
A: The DHCoE supports and encourages developers—including those who are new to the health care space—to translate digital advances into tools that benefit consumers. This is accomplished through the wide array of services offered, including developing a readily accessible knowledge hub and new strategic partnerships. The DHCoE is creating a network of digital health experts to share knowledge and expertise with the FDA.
The DHCoE is also working to tailor the FDA’s oversight of digital health technology by reimagining its regulatory approach to Software as a Medical Device (SaMD) (for example, Software Precertification Pilot Program), as well as exploring how to leverage the unique benefits of Artificial Intelligence and Machine Learning in SaMD.
The DHCoE will also provide ongoing clarity for policy and regulation, and work to coordinate international harmonization of digital health regulation.
Q: Who should I contact if I have questions or would like to collaborate with the DHCoE?
A: To engage the DHCoE, send an email to digitalhealth@fda.hhs.gov.
Q: How is the DHCoE structured?
A: The DHCoE aligns and coordinates digital health work across the FDA.
- The DHCoE is based within the FDA’s Center for Devices and Radiological Health (CDRH).
- To align strategies within CDRH, the DHCoE coordinates the Digital Health Steering Committee, which sets CDRH’s agenda for digital health policy, external partnerships, regulatory science, and regulatory needs.
- To align strategies within the FDA, the DHCoE will work to coordinate digital health projects across the FDA’s Centers by identifying common interest areas including aligning on the FDA-wide agenda for digital health regulatory science.
Q: Is the DHCoE part of the Technology Modernization Action Plan?
A: The DHCoE complements and is coordinated with the Technology Modernization Action Plan. The DHCoE will primarily focus on regulatory oversight and related topics while the Technology Modernization Plan will focus on the technological infrastructure modernization for the agency.
Q: I’m interested in working at the DHCoE. What positions are available?
A: We are seeking software engineers, artificial intelligence and machine learning engineers, security researchers, user interface and user experience designers, and product managers. Learn more about the roles and how to apply.