Digital Health Center of Excellence Services
The Digital Health Center of Excellence (DHCoE) serves to complement advances in digital health technology. To accomplish this, we provide services to digital health stakeholders—including those who are new to the health care space—to translate digital advances into tools that benefit consumers. We offer support in the following service areas:
Empowering Stakeholders
The Digital Health Center of Excellence empowers digital health stakeholders to advance health care by fostering responsible and high-quality digital health innovation by:
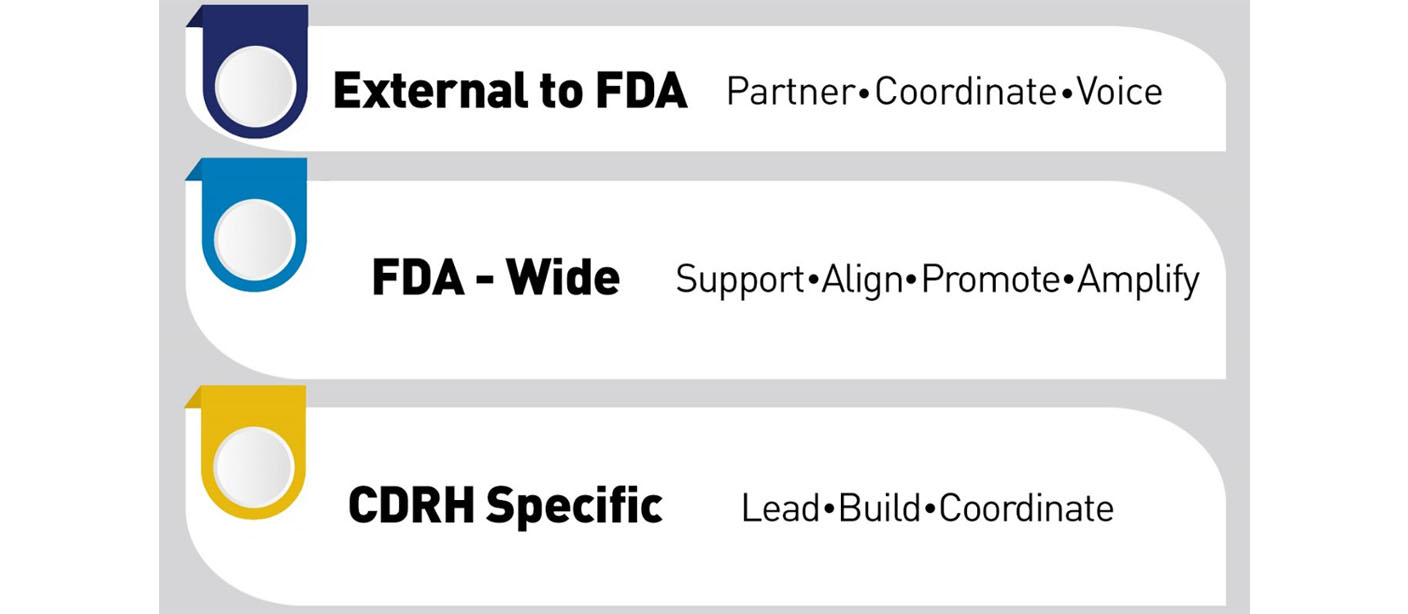
- Setting and leading strategic direction in digital health technology at the Center for Devices and Radiological Health (CDRH)
- Launching strategic initiatives that advance digital health technologies
- Building new capacity to oversee and leverage digital health technologies
- Providing scientific expertise across the FDA
- Providing technological and policy advice to support the FDA decision-making processes
- Promoting and showcase existing work across the FDA
- Transparently share resources for developers
Examples include:
- Artificial Intelligence / Machine Learning Discussion Paper
- Cybersecurity
- NEST Demonstration Project Post-Market Medical Device Surveillance with a Novel mHealth Platform
Connecting Stakeholders
The Digital Health Center of Excellence connects and builds partnerships to accelerate digital health advancements by:
- Fostering collaboration across the FDA in common interest areas
- Facilitating synergies in regulatory science research in digital health
- Facilitating and building strategic partnerships
- Communicating the FDA’s research interests
- Advancing international harmonization on device regulatory policy
- Advancing digital health technology international standards
- Providing access to internal and external digital health experts
Examples include:
- Network of Digital Health Experts
- Ophthalmic Imaging Collaborative Community
- IMDRF Medical Device Cybersecurity Guide
- IMDRF Artificial Intelligence Medical Devices (AIMDs)
Sharing Knowledge
The Digital Health Center of Excellence shares knowledge to increase awareness and understanding, drive synergy, and advance best practices by:
- Sharing information to increase awareness of advancements in digital health
- Establishing and promoting best practices
- Creating and disseminating shared resources internally and externally
- Offering training opportunities for the FDA’s staff and external stakeholders
- Communicating the FDA’s research interests
Examples include:
- Guidances with Digital Health Content
- What is a SaMD?
- Device Software Functions Including Mobile Medical Applications
Innovating Regulatory Approaches
The Digital Health Center of Excellence innovates regulatory approaches to provide efficient and least burdensome oversight by:
- Enabling efficient, transparent, and predictable product review with consistent evaluation quality
- Providing clarity on regulation by developing cross-cutting digital health guidance
- Developing novel, efficient medical device regulatory approaches that are least burdensome while meeting FDA standards
Examples include: