Edwards SAPIEN 3 Transcatheter Pulmonary Valve System with Alterra Adaptive Prestent – P200015/S011
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: Edwards SAPIEN 3 Transcatheter Pulmonary Valve System with Alterra Adaptive Prestent
PMA Applicant: Edwards Lifesciences LLC
Address: One Edwards Way, Irvine, CA 92614
Approval Date: August 31, 2020
Approval Letter: Link to Approval Order
What is it?
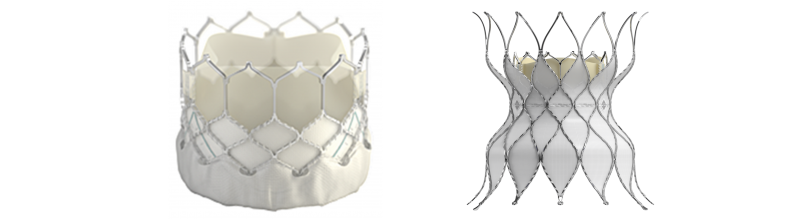
The Edwards SAPIEN 3 Transcatheter Pulmonary Valve System with Alterra Adaptive Prestent consists of a catheter-based stent (named Alterra), an artificial heart valve (named SAPIEN 3) and accessories used to implant the stent and valve without open-heart surgery. The stent is made from self-expanding nickel-titanium (Nitinol). The valve is made of cow tissue attached to a balloon-expandable, cobalt-chromium frame for support.
The Edwards SAPIEN 3 Valve System was previously approved for pulmonary valve replacement when a patient’s pulmonary valve conduit (an artificial tube with a valve inside that connects the heart to the lungs) or artificial pulmonary valve stops working properly. This approval expands use of this device in patients whose right ventricular outflow tract (RVOT; the part of the heart that carries blood to the lungs) becomes too leaky (blood flows in the wrong direction), a condition that often results from congenital heart disease. These are patients who have not had their RVOT replaced by a pulmonary valve conduit and either have not had a prior surgery to repair their RVOT (native RVOT) or have had a prior surgery to do so (surgically repaired RVOT).
How does it work?
The doctor first inserts a tube-like device called catheter through a large vein in the leg. The cathether has a compressed Alterra stent at its end. The catheter is pushed through the blood vessels until it reaches the pulmonary valve. The stent is released and anchors to the patient’s RVOT. Another tube-like device called a balloon catheter is then inserted through the same place in the leg. This catheter has a compressed SAPIEN 3 valve at its end. It is pushed through the blood vessels until it reaches the Alterra stent. The artificial valve is then expanded by a balloon, and it anchors inside the Alterra stent. Once the new valve is in place, it functions the same as the old valve, opening and closing like a door to force the blood to flow in the correct direction.
When is it used?
The Edwards SAPIEN 3 Valve System is used to replace a failing pulmonary valve in patients born with heart defects when their native or surgically repaired RVOT becomes severely leaky and needs to be replaced. The SAPIEN 3 valve can be used as the replacement valve without open heart surgery.
What will it accomplish?
The SAPIEN 3 Valve System may be used in place of open-heart surgery for a valve replacement, giving the patient more time between open heart surgeries or reducing the total number of open-heart surgeries the patient needs.
In a clinical study of the SAPIEN 3 valve and Alterra stent, none of the SAPIEN 3 valves implanted stopped working as intended at six months. Complications reported in the clinical study included irregular heartbeat (34 out of 100 patients), bleeding (22 out of 100 patients, including 19 out of 100 patients with minor bleeding), and minor injury to the blood vessels or complication at the site in the leg where the catheters were inserted (5 out of 100 patients).
When should it not be used?
The SAPIEN 3 valve and Alterra stent should not be used in patients who:
- Cannot tolerate blood thinning medicines
- Have an infection in the heart or elsewhere