CTPConnect— Winter 2021
This quarterly newsletter serves as a digest of the latest announcements and stories out of CTP. It is a complement to our Spotlight on Science newsletter and CTP News e-blasts.
A 2020 Message from Former FDA Commissioner Dr. Stephen Hahn
Despite the unforeseen challenges posed by the COVID-19 pandemic, it was a year of unparalleled contributions to public health at FDA. In a review of the agency’s initiatives and achievements, Former FDA Commissioner Dr. Stephen Hahn offers a highlight of what was accomplished, including the Center for Tobacco Products’ comprehensive, science-based approach to regulating an evolving tobacco landscape and protecting the public – especially youth – from the harms associated with tobacco product use.
CTP continued to demonstrate our commitment to this mission over the last year by completing historic regulations, reviewing tobacco product premarket applications using rigorous scientific standards, enforcing the law and our regulations, and continuing our award-winning public education campaigns.
In This Issue:
Regulation and Guidance:
- Postponed: Cigarette Health Warnings Effective Date
- FDA Issues Guidance on Tobacco Product Perception and Intention Studies
- FDA Updates Deeming Guidance to Reflect Recent Court Decisions
Compliance and Enforcement:
Public Health and Education:
- Youth Use of Tobacco Products Decreased in 2020, National Survey Finds
- Virtual Learning: Youth E-Cigarette Prevention Magazine, Contest and Blog
- Virtual Exhibit Booth Experience and Free Tobacco Education Materials
Other Recent Announcements:
- FDA Issues Marketing Order for IQOS 3 System Holder and Charger
- FDA Releases Updated Version of the Safety Reporting Portal’s Tobacco Questionnaires
- Did You Know…?
Regulation and Guidance
Postponed: Cigarette Health Warnings Effective Date
On December 2, 2020, the U.S. District Court for the Eastern District of Texas granted a motion by the plaintiffs in the case of R.J. Reynolds Tobacco Co. et al. v. United States Food and Drug Administration et al., No. 6:20-cv-00176, to postpone the effective date of the “Required Warnings for Cigarette Packages and Advertisements” final rule by an additional 90 days.
The new effective date of the final rule is January 14, 2022. Pursuant to the court order, any obligation to comply with a deadline tied to the effective date is similarly postponed.
FDA strongly encourages entities to submit cigarette plans as soon as possible, and in any event by March 16, 2021. FDA has revised the “Submission of Plans for Cigarette Packages and Cigarette Advertisements” guidance and “Required Warnings for Cigarette Packages and Advertisements” small entity compliance guide to include the rule’s new effective date and updated the recommended date for submission of cigarette plans.
FDA Issues Guidance on Tobacco Product Perception and Intention Studies
On Oct. 27, 2020, FDA issued the draft guidance, “Tobacco Products: Principles for Designing and Conducting Tobacco Product Perception and Intention Studies.” This draft guidance describes the agency’s proposed recommendations on designing and conducting tobacco product perception and intention (TPPI) studies that may be submitted as part of a tobacco product application, such as a modified risk tobacco product (MRTP) application, premarket tobacco product application (PMTA), or substantial equivalence (SE) report.
TPPI studies are studies that can be used to assess, among other things, individuals’ perceptions of tobacco products, understanding of tobacco product information (e.g., labeling, modified risk information), and intentions to use tobacco products. Among other things, the draft guidance provides an overview of general scientific principles to consider regarding TPPI study design and methods. It also includes scientific considerations related to developing study aims and hypotheses, selecting appropriate measures and study outcomes, selecting and justifying study samples, collecting data, and analyzing and reporting study results.
While not legally binding, the information in the draft guidance is intended to help applicants prepare TPPI studies that can help demonstrate that a tobacco product meets the applicable marketing authorization standard.
FDA Updates Deeming Guidance to Reflect Recent Court Decisions
On October 16, 2020, FDA posted a revised guidance to reflect recent court decisions regarding FDA’s enforcement of premarket requirements for premium cigars, as well as health warning and labeling requirements for cigars and pipe tobacco.
On Aug. 19, 2020, the U.S. District Court for the District of Columbia issued a ruling that, in part, prohibits FDA enforcement of the Tobacco Control Act’s premarket authorization requirement for premium cigars until after the agency considers developing a streamlined substantial equivalence process specifically for premium cigars.
Additionally, on Sept. 11, 2020, the D.C. Circuit court vacated the health warning requirements for cigars and pipe tobacco, remanding the issue back to FDA to more closely consider whether the health warnings would likely affect the number of cigars and pipe tobacco users. FDA will not seek to enforce the applicable warning and labeling requirements for cigars and pipe tobacco at this time.
Accordingly, FDA revised the “FDA Deems Certain Tobacco Products Subject to FDA Authority, Sales and Distribution Restrictions, and Health Warning Requirements for Packages and Advertisements (Revised)*” Small Entity Compliance Guide to reflect these court orders. Aside from the revisions to reflect these court decisions, no other changes were made to this guidance.
Compliance and Enforcement
FDA Warns Firms to Remove Unauthorized E-liquids from Market in Letters Issued to Manufacturers that Did Not Submit Premarket Applications by Deadline
Starting in January, FDA issued warning letters to firms who have not submitted premarket applications to FDA and are continuing to sell or distribute unauthorized electronic nicotine delivery system (ENDS) products after Sept. 9, 2020. As of now, FDA has issued warning letters to 30 firms who manufacture and sell e-liquids, advising them that selling these products, which lack premarket authorization, is illegal, and therefore they cannot be sold or distributed in the U.S.
Per court order, applications for premarket review for certain deemed new tobacco products on the market as of Aug. 8, 2016—including e-liquids—were required to be submitted to FDA by Sept. 9, 2020. For companies that submitted applications by that deadline, FDA generally intends to continue to defer enforcement for up to one year pending FDA review, unless there is a negative action taken by FDA on the application. FDA plans to post a list of products for which the agency has received applications; however, before making such a list available, FDA is verifying certain information about these products so that publication of a list complies with federal disclosure laws.
While each warning letter issued cites specific products as examples, collectively these companies have listed a combined total of over 350,000 products with FDA. Companies must ensure all of their products comply with federal rules and regulations—including the premarket review requirement.
Public Health and Education
Youth Use of Tobacco Products Decreased in 2020, National Survey Finds
In December 2020, FDA, in partnership with CDC, released new data from the 2020 National Youth Tobacco Survey (NYTS). The data show youth use of any tobacco product declining, with an estimated 1.73 million fewer youth currently using any tobacco product in 2020 (4.47 million) compared to 2019 (6.20 million).
The results showed 23.6% of high school and 6.7% of middle school students reported currently using any tobacco product in 2020. In addition, current use of any combustible tobacco products, multiple tobacco products, e-cigarettes, cigars, and smokeless tobacco decreased among both high and middle school students between 2019 and 2020. Despite these encouraging declines, the survey did not show significant decreases in the use of cigarettes, hookah, pipe tobacco, or heated tobacco products.
The latest data follow previously released findings from the 2020 NYTS focused on youth use of e-cigarettes.
Virtual Learning: Youth E-Cigarette Prevention Magazine, Contest and Blog
FDA continued to work with Scholastic to develop youth e-cigarette prevention materials for middle and high school students. A student magazine and an accompanying teacher guide are now available to teach students about the risks of e-cigarette use and nicotine addiction.
Scholastic also launched The “Vaping’s Not My Thing” Student Challenge. Students are asked to create a poster that convinces teens not to vape because of the health risks. Entries will be accepted until March 22, 2021 and will be judged on, among other things, scientific content and persuasiveness. Prizes will be funded by Scholastic and awarded in spring 2021. Kathy Crosby, Director of Communications at FDA’s Center for Tobacco Products, has also authored blogs which advise parents and teachers to be on the lookout for disposable and stealth e-cigarettes and inform them about the latest facts on youth use.
Additional Scholastic materials include lesson plans, activity sheets, a guide for parents to talk to kids about e-cigarettes, and infographics. These materials are accessible online for free and are adaptable for remote instruction or independent student work.
As the school year continues, many students are still attending virtual or modified school schedules, which can be incredibly difficult for families and children. To help address these challenges, Scholastic grounded the materials in core subjects and weaved in activities that can easily be done from home, ensuring that these digital materials are still engaging and accessible.
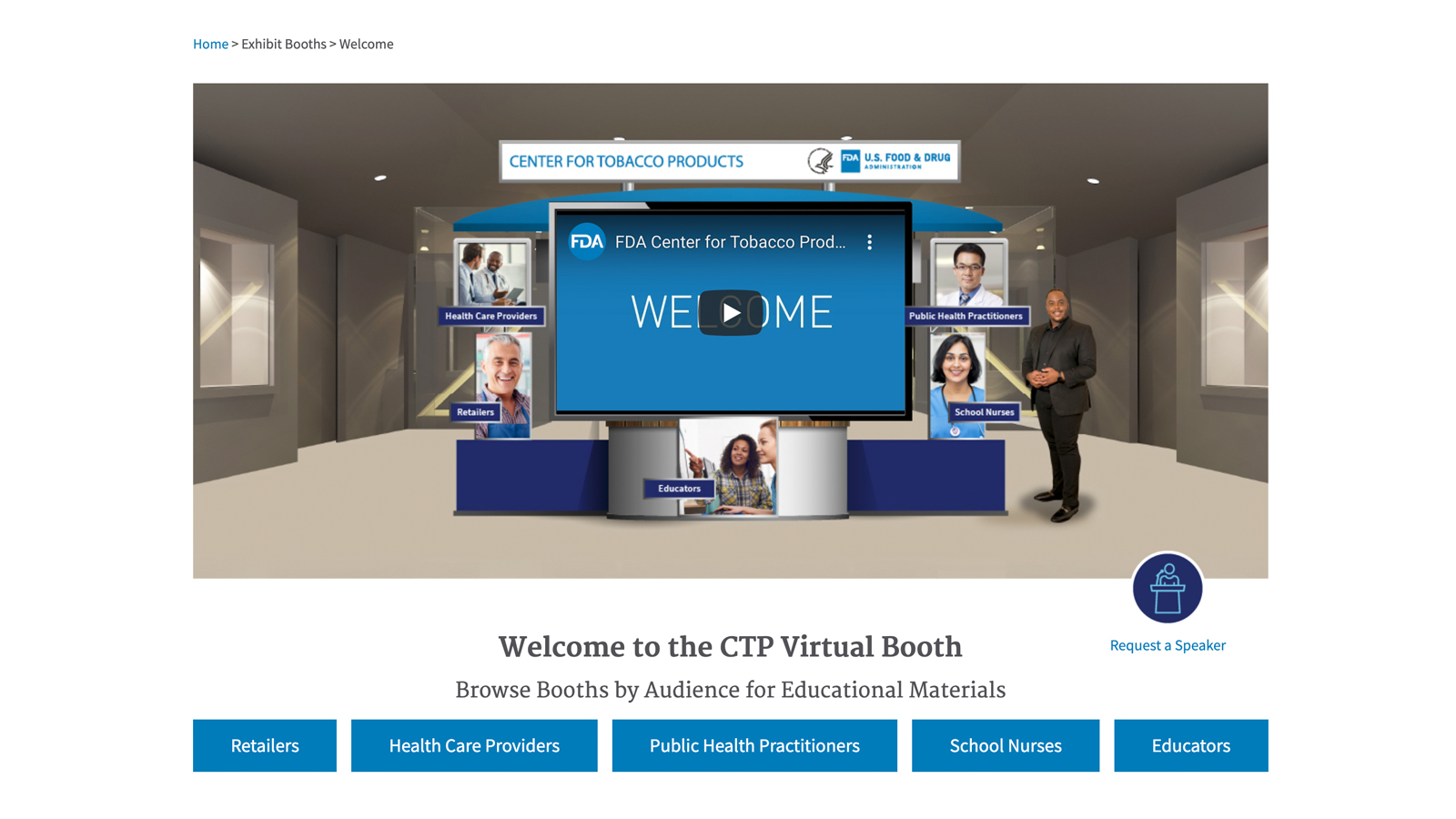
Virtual Exhibit Booth Experience and Free Tobacco Education Materials
FDA recently launched a virtual exhibit booth, which provides visitors an opportunity to explore CTP resources on the Exchange Lab. This virtual platform provides a “site within a site” series of carefully curated and tailored landing pages (with a unique URL) designed for several of CTP’s targeted stakeholder audiences - public health practitioners, health care providers, school nurses, educators, and tobacco retailers.
The Exchange Lab provides free print materials, web content and social media posts to help keep communities informed about tobacco-related issues. It offers a wide variety of science-based content focused on public health education, tobacco research, retailer information, and tobacco regulations and compliance.
Other Recent Announcements
FDA Issues Marketing Order for IQOS 3 System Holder and Charger
On Dec. 7, 2020, FDA issued a marketing order to Philip Morris Products S.A. authorizing the sale of the IQOS 3 System Holder and Charger. Compared to the previous version authorized in April 2019, the newly authorized version has minor design differences, including how the holder inserts into the charger, changes to the charging connectors and LED indicator lights, a new touch feedback feature, and an option to reduce the perceived heat from the tobacco aerosol inhaled by users.
Data on product use in international markets suggest no differences among user populations from the previous version of the device holder and charger, including no new concerns regarding product initiation or use among youth and young adults. Following FDA’s scientific review of the application, the agency found, among other things, that the modifications to the device do not raise new concerns related to safety, health effects, product quality, or product misuse.
Importantly, this action does not mean this product is safe or “FDA-approved.” There are no safe tobacco products, and those who do not use tobacco products should not start. The previous version of the IQOS System Holder and Charger was initially allowed for sale in the United States following FDA’s issuance of a marketing order via the premarket tobacco product application (PMTA) pathway in April 2019. The company later submitted another PMTA to FDA in April 2020 requesting authorization to market an updated version of the device holder and charger.
This is the first “supplemental” PMTA received by FDA. Such applications seek authorization to modify a tobacco product that previously received a marketing order via the PMTA pathway. “Supplemental” PMTAs must still contain the information required by section 910(b)(1) of the Federal Food, Drug, and Cosmetic Act, but they may meet these requirements by cross-referencing content from the relevant previously authorized PMTA and by providing new information related to the product modifications.
FDA Releases Updated Version of the Safety Reporting Portal’s Tobacco Questionnaires
FDA has made several updates to the Safety Reporting Portal (SRP) questionnaires to better capture information about affected individuals, pre-existing health conditions, and product-specific details.
For example:
- All voluntary reports must now include the affected person’s age as well as how many people were affected, and whether nonusers were affected.
- E-cigarette or vape users are asked how much nicotine is in the tobacco product that seemed to cause the problem.
- Optional questions about health problems have been expanded to assess specifically for physical or mental disabilities and English language ability.
- Optional questions related to e-cigarette or vape products now request more information about the product’s battery, its operating status, and other details to help identify the specific product involved.
For researchers providing voluntary reports of adverse experiences that occurred during a study, information on expectedness and relatedness of the problem to the study tobacco product is now required. Research group accounts can also add multiple e-mail addresses to be notified when a report is submitted.
FDA encourages anyone who has had a reaction to, or was hurt by, a tobacco product – or knows someone who experienced such effects – to visit the SRP and provide as much information as possible. Reports may be submitted anonymously, but including contact information allows FDA the option to find out more about the adverse experience. FDA reviews all tobacco-related SRP reports to identify new or concerning trends.
Did You Know…
… you can request a speaker from CTP’s Speakers Bureau, a centralized place to request expert speakers on CTP’s regulatory actions and public education initiatives?
To request a speaker from CTP’s Speakers Bureau, submit the following to CTPSpeakerRequests@fda.hhs.gov:
- A completed speaker request form;
- A formal invitation on organization letterhead, and;
- A program agenda with all invited speakers and topics.
For more information, please visit our Speakers Bureau webpage or contact us at CTPSpeakerRequests@fda.hhs.gov.