Grandfathered Tobacco Products
On this page:
- Overview of Grandfathered Tobacco Products
- Search Grandfathered Determinations
- Preparing a Voluntary Grandfathered Status Determination Request
- Voluntary Grandfathered Tobacco Product Status Determination Program
- Grandfathered Tobacco Product Resources
Overview of Grandfathered Tobacco Products
A grandfathered tobacco product* is a tobacco product that was commercially marketed in the United States as of February 15, 2007. The FDA interprets “as of” to mean “on” that date. Such tobacco products are regulated under the Federal Food, Drug, and Cosmetic Act (FD&C Act) and do not require premarket authorization to be legally marketed.
* In the PMTA final rule and SE final rule, FDA has changed the term “grandfathered tobacco product” to “Pre-Existing Tobacco Product” because it more appropriately describes these products. Specifically, FDA considers a “Pre-Existing Tobacco Product” to mean a tobacco product that was commercially marketed in the United States as of February 15, 2007.
Search Grandfathered Determinations
FDA's standalone grandfathered submissions database contains grandfathered determination information from voluntarily-submitted requests for a grandfathered status determination of a tobacco product.
This database does not list all grandfathered tobacco products. It is updated periodically and contains original submission information that is typically released in response to a FOIA request.
Preparing a Voluntary Grandfathered Status Determination Request
Submitting a request to determine the grandfathered status of your tobacco product is voluntary and not required under the FD&C Act. A grandfathered tobacco product is not considered a new tobacco product and does not need premarket authorization to be legally marketed.
There are three main components of a request to determine a tobacco product’s grandfathered status:
- A description of the product, including the name it was commercially marketed under as of February 15, 2007, and characteristics that uniquely identify it.
- A statement that the product was commercially marketed, not exclusively in a test market, in the United States as of February 15, 2007.
- Dated evidence that shows the product was commercially marketed in the United States as of February 15, 2007.
When preparing a request to determine a tobacco product’s grandfathered status, be sure to complete a separate grandfathered submission for each tobacco product.
Unable to Accept (UTA) Letters: For grandfathered submission requests received on or after January 15, 2020, FDA will issue UTA letters to industry if a grandfathered status determination request is not submitted individually. FDA will not accept or review submission requests if they contain multiple products for which a firm is seeking grandfathered status determinations. Each product must be submitted in an individual submission request either electronically via CTP Portal using FDA’s eSubmitter or mailed to CTP’s Document Control Center (DCC).
Every submission should include the following:
- Submission content or evidence in English.
- Labeled as a “Grandfathered Submission.”
- Applicant name (may be the manufacturer, distributor, or importer of the tobacco product) and contact information.
- Full exact name, including brand and sub-brand, of the tobacco product as it was commercially marketed in the United States on February 15, 2007. Keep naming consistent throughout the submission.
- Unique identifying characteristics of the product, such as package type, quantity, length, diameter, tobacco cut size, portion mass, and flavor.
- Demonstrate the product was not exclusively marketed in test markets. This confirmation may be submitted in the form of a statement from a responsible official who has knowledge of the test marketing status of the tobacco product in the United States as of February 15, 2007, and has authority to make such a statement.
- Evidence that the product was commercially marketed in the United States as of February 15, 2007. All evidence of commercial marketing should be dated and include the product’s full name. Examples of documentation of commercial marketing include advertisements, catalog pages, promotional material, trade publications, bills, invoices, purchase orders, inventory lists, receipts, and manufacturing documents.
Unable to Review (UTR) Letters: For submission requests received on or after January 15, 2020, FDA will issue UTR letters to industry if a grandfathered status determination request does not include sufficient information for FDA to find that the tobacco product is grandfathered, and continued agency review of the submission would be an inefficient use of agency resources. Under these circumstances, FDA will issue a UTR letter to the firm and close-out the submission request and Submission Tracking Number (STN). The UTR letter will describe the basis for issuing the UTR letter and inform the firm that they may resubmit their grandfathered submission, at any time. Firms will receive UTR letters if the grandfathered submission contains any one of the following problems:
- Grandfathered submission is missing two or more of the following pieces of information:
- test marketing information
- commercial marketing evidence
- unique product identifiers
-
Grandfathered submission or evidence contained in the submission is not translated into English.
-
FDA determines that the specific product identified in the grandfathered submission is not a tobacco product.
-
Grandfathered submission lacks firm/manufacturer name and contact information.
How to submit a request for a grandfathered status determination
- Online
- Request an Industry Account Manager (IAM) account to establish a CTP Portal account, if your company does not already have an IAM.
- Prepare your submission electronically using FDA's eSubmitter software.
- Submit online via the CTP Portal.
Note: The CTP Portal provides more functionality than the ESG, and CTP recommends using the CTP Portal. However, if you already have an ESG WebTrader account, you may use it to submit documents to CTP.
- By Mail: If you are unable to submit online, mail submissions to CTP's Document Control Center.
For specific questions about an existing grandfathered submission, please contact CTP-Grandfather@fda.hhs.gov. Be sure to reference the assigned STN in the inquiry.
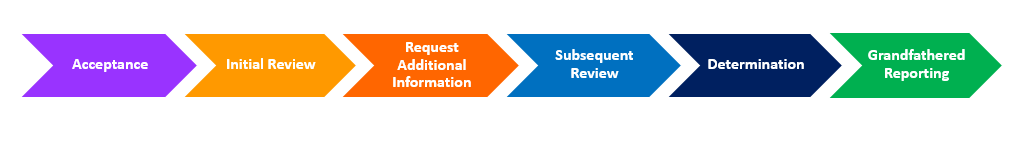
Voluntary Grandfathered Tobacco Product Status Determination Program
- Request received:
- Output:
- Acknowledgement letter with Submission Tracking Number (STN) and the official date of receipt
- Unable to Accept (UTA) Letters will be issued for review submission requests if they contain multiple products for which a firm is seeking grandfathered status determinations. Each product must be submitted in an individual submission request either electronically via CTP Portal using FDA’s eSubmitter or mailed to CTP’s Document Control Center (DCC).
Note: Unable to Accept letters are not final agency action and do not preclude a firm from providing a revised Grandfathered submission.
- Output:
- Review: FDA reviews the submission to determine if it meets the definition of a Grandfathered tobacco product. During this process, FDA cannot provide a specific date by which the review will be completed.
- Output:
- Unable to Review (UTR) letter may be issued for several reasons:
- Grandfathered submission is missing two or more of the following pieces of information:
- test marketing information
- commercial marketing evidence
- unique product identifiers
- Grandfathered submission or evidence contained in the submission is not translated into English.
- FDA determines that the specific product identified in the grandfathered submission is not a tobacco product.
- Grandfathered submission lacks firm/manufacturer name and contact information.
Note: Unable to Review letters are not final agency action and do not preclude a firm from providing a revised Grandfathered submission; however, that submission will be assigned a separate STN number and undergo a new review.
- Grandfathered submission is missing two or more of the following pieces of information:
- Request for Information (RFI) letter to obtain additional information or evidence necessary to complete the review. Firms will have 30 calendar days to respond to the RFI letter. Only one RFI letter will be sent. No extensions to this timeline are typically granted.
- Unable to Review (UTR) letter may be issued for several reasons:
- Output:
- Determination:
- Output
- Grandfathered Status Determination letter: Based on the information submitted, FDA determines that the tobacco product is Grandfathered. The tobacco product is added to the FDA’s Standalone Grandfather Determination Database including the name of the tobacco product as commercially marketed in the United States on February 15, 2007, category of product, and the name of the company/request submitter.
- Unable to Grandfather Determination letter: Based on the information submitted, FDA cannot determine that the tobacco product is Grandfathered.
Note: Unable to Grandfather letters are not final agency action and do not preclude a firm from providing a revised Grandfathered submission; however, that submission will be assigned a separate STN number and undergo a new review.
- Output
For specific questions about your existing Grandfathered submissions, please contact CTP-Grandfather@fda.hhs.gov. Be sure to reference the assigned STN in the inquiry.
Grandfathered Tobacco Product Resources
- Guidance: Establishing that a Tobacco Product was Commercially Marketed in the United States as of February 15, 2007
- Video: Standalone Grandfathered Submissions
- Memo: Unique Identification of Tobacco Products
- Substantial Equivalence Marketing Orders: Information provided within these orders may also contain information on predicate products that are also grandfathered
- Q&A: Tobacco Product Review and Evaluation and Pathways to Market
- For specific questions about your existing Grandfathered submissions, please contact CTP-Grandfather@fda.hhs.gov. Be sure to reference the assigned STN in the inquiry.