Developing Predictive Indicators of Genome Stability and Cell Maturation as Measures of Cell Therapy Product Safety and Efficacy
Deborah Hursh, Ph.D.
Office of Tissues and Advanced Therapies
Division of Cellular and Gene Therapies
Cellular and Tissue Therapy Branch
Biosketch
Deborah Hursh is a senior investigator and Chemical, Manufacturing, and Control (CMC) reviewer in the Division of Cellular and Gene Therapies, Office of Tissues and Advanced Therapies at the Center for Biologics Research and Review (CBER) of FDA. Dr. Hursh received a Ph.D. in Molecular, Cellular and Developmental Biology from Indiana University and did postdoctoral work in the Dept. of Cellular and Developmental Biology at Harvard University. She was a senior staff fellow in the Laboratory of Biochemistry at the National Cancer Institute of the National Institutes of Health, and an assistant professor of Biology at American University (Washington, D.C.) prior to moving to CBER in 2000. Her expertise is in cell and developmental biology. At FDA, Dr. Hursh evaluates a wide range of products relevant to cell and gene therapies and participates in policy development in the areas of stem cells and assisted reproduction. She was the chair of the Humanitarian Device Exemption (HDE) review committee, which approved the Miltenyi CliniMACS CD34 Reagent System, a Class III device used to select stem cells. She was chair of the organizing committee for a CBER advisory meeting in 2014 on oocyte and embryo modification in assisted reproduction for the prevention of transmission of mitochondrial disease or treatment of infertility. In addition to her regulatory activities, she directs a research lab studying issues relevant to the safety and effectiveness of cell therapy products.
General Overview
Living cells are being used directly, and to engineer tissues, to repair and regenerate diseased or damaged tissues. In order for the therapies derived from this promising field of medical research to be safe and effective, scientists must learn how to reliably predict the behavior of these cellular products after they are administered to patients. Cells that do not grow and mature in predictable ways will not provide effective treatment; they can also cause serious adverse consequences, such as tumors.
After a cell therapy product is administered to a patient, the fate of those cells depends largely on their ability to successfully communicate with their environment. Both the cells and their host environment carry out this communication by releasing signaling molecules called growth factors. These growth factors work together and guide the maturation of the transplanted cells so they can repair tissues.
To ensure that cell and tissue therapy products will work effectively, this research program seeks to de-code the complex communications used by cells during maturation. The program uses a simple organism as a model to study communication among cells. We focus on communication networks known to be active in embryonic development and regeneration: the Bone Morphogenetic Protein, Wnt, and Hedgehog pathways. We are able to screen all the genes (complete genome) of our organism to identify molecules involved in cell-cell communication networks. By this method, we identify genes that can serve as predictive indicators of cell fate. The cell communication networks we study have been shown to be identical among all animals, so the data acquired in our model system will be applicable to humans.
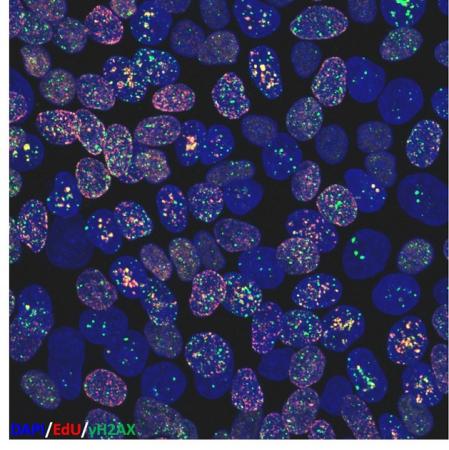
We also study how the manufacture of cellular and tissue products affects the quality of human cells. Cells that develop genetic damage (e.g., mutations) during manufacture can cause tumors when transplanted, or they may not perform in the desired way. This is particularly a problem with immature cells such as stem cells. Therefore, we are working to develop methods to assess genome stability in cultured stem cells. We use methods such as karyotyping to monitor damage to whole chromosomes, but are also investigating methods that may provide early indication of genome damage. One method is to use a chromatin protein modification which appears at sites of DNA breakage to identify DNA damage as an early marker of culture conditions that impact genetic stability. We are also examining the energy producing organelles of the cell, mitochondria, to see if they can serve as sentinels of cells under manufacturing stress. Furthermore, we are exploring how in vitro culture conditions, such as oxygen level, or 2-dimensional versus 3-dimensional culture, affect genome stability and mitochondrial behavior.
Developing markers that help to predict the fate, behavior, and genetic integrity of cells is critical to our role in ensuring the safe and effective use of all cell therapy products, particularly in the emerging area of stem cell therapy. Lack of identified markers is a current hurdle to the development of safe and effective cell therapies.
Scientific Overview
Part of this research program focuses on the role played by intercellular communication by peptide growth factors in cell differentiation and tissue formation. Using the tools of genetics and molecular biology, our goal is to describe biochemical pathways through which growth factors act to modulate morphology, gene expression, and cell behavior. Our major focus is the Bone Morphogenetic Protein (BMP) pathway. BMPs are members of the Transforming Growth Factor beta family. Results are relevant to the use of growth factors as cytokines in the manufacture of cellular and tissue products, as well as therapeutic agents in and of themselves. BMP-mediated signaling facilitates in vitro or in vivo cell maturation and functions in the stem niche.
Furthermore, understanding the stability of differentiated cells and their capacity to be reprogrammed by their environment is a major concern in cellular therapies, particularly those using stem cells or engineered tissues. We use the fruit fly Drosophila melanogaster as a model system. Drosophila is a well-established system for analyzing protein function via rapid, genome-wide in vivo screening. All signal transduction pathways (TGF-beta, Wnt, Hedgehog, FGF, EGF, MAP kinase, JNK kinase) are highly conserved between Drosophila and mammals; therefore results from Drosophila can be extended to humans. However, where mammals often have multiple genes for individual signal transduction pathway members, the simpler fruit fly typically has single gene representatives of critical signaling proteins. Thus the issue of genetic redundancy, which often obscures genetic analysis in mammals, is avoided.
The lab has developed a sensitive genetic model of BMP activity to do genetic interaction screens that identify markers of growth factor activity. Using these screens, we identify proteins whose expression correlates with BMP activity and examine their relationship with BMP action. Using this screen, we discovered previously unknown interactions between the BMP pathway and other critical growth factor pathways.
The lab is also exploring measures of genetic and phenotypic stability in cultured human stem cells as predictive indicators of cell quality. For genetic stability we examine chromosomes and histone chromatin proteins. We examine chromosome integrity at the single cell level by spectral karyotyping. Cells change the post-translational modifications of histone proteins in response to environmental stresses, and these changes may provide leading indicators of genetic damage and environmental toxicity. We are investigating histone modifications created at sites of DNA damage to obtain a genome-wide snapshot of genetic integrity in stem cells and their differentiated progeny. Genetic regions bearing this modification can be identified using chromatin immunoprecipitation (ChIP), a method that locates histone post-translational modifications associated with specific genes. ChIP can be carried out genome-wide (ChIP-Seq), using whole-genome deep sequencing.
In addition, the lab evaluates mitochondrial characteristics as predictive measures of phenotypic stability in stem cells. Mitochondria respond to their environment, such as culture conditions, by altering their morphology, number per cell, and energy output. Mitochondrial attributes may therefore predict cell behavior.
The latter projects are examining the effects of in vitro culture manipulations commonly used by cell therapy manufacturers on the genetic stability and behavior of stem cells.
Important Links
Publications
- Methods Mol Biol 2019;1891:75-89
Gene regulation of BMP ligands in Drosophila.
Stultz BG, Hursh DA - Stem Cells 2018 Oct;36(10):1501-13
High basal levels of gammaH2AX in human induced pluripotent stem cells are linked to replication-associated DNA damage and repair.
Vallabhaneni H, Lynch PJ, Chen G, Park K, Liu Y, Goehe R, Mallon BS, Boehm M, Hursh DA - Adv Exp Med Biol 2018;1046:41-58
Odd-paired: the Drosophila Zic gene.
Hursh DA, Stultz BG - Elife 2017 Mar 21;6:e17935
SMOC can act as both an antagonist and an expander of BMP signaling.
Thomas JT, Eric Dollins D, Andrykovich KR, Chu T, Stultz BG, Hursh DA, Moos M - Fly 2016 Oct;10(4):195-203
Jun N-terminal kinase signaling makes a face.
Hursh DA, Stultz BG, Park SY - Cytotherapy 2016 Sep;18(9):1114-28
In vitro cytokine licensing induces persistent permissive chromatin at the Indoleamine 2,3-dioxygenase promoter.
Rovira Gonzalez YI, Lynch PJ, Thompson EE, Stultz BG, Hursh DA - Cytotherapy 2016 Mar;18(3):336-43
Chromosomal stability of mesenchymal stromal cells during in vitro culture.
Stultz BG, McGinnis K, Thompson EE, Lo Surdo JL, Bauer SR, Hursh DA - Genetics 2015 Dec;201(4):1411-26
Dual role of Jun N-terminal kinase activity in bone morphogenetic protein-mediated Drosophila ventral head development.
Park SY, Stultz BG, Hursh DA - Stem Cells 2015 Jul;33(7):2169-81
Chromatin changes at the PPAR-γ2 promoter during bone marrow-derived multipotent stromal cell culture correlate with loss of gene activation potential.
Lynch PJ, Thompson EE, McGinnis K, Rovira Gonzalez YI, Lo Surdo J, Bauer SR, Hursh DA