Impact of Immunity Induced by Viral and DNA Products

Suzanne L. Epstein, Ph.D.
Office of Tissues and Advanced Therapies
Division of Cellular and Gene Therapies
Gene Transfer and Immunogenicity Branch
Biosketch
Suzanne Epstein received her BA. in chemistry from Harvard University in 1972 and her PhD in biology from MIT in 1979. Following postdoctoral research in immunology at the National Cancer Institute, she came to FDA in 1985 as a PI and regulatory reviewer, at what was then the Center for Drugs and Biologics. She is currently Associate Director of Research for the Office of Tissues and Advanced Therapeutics at CBER, in addition to performing research and regulatory review.
While at FDA she has reviewed diverse investigational new drug (IND) applications (especially cellular and gene therapies), served on a licensing committee and inspection team, and developed cell and gene therapy policy (lead author, “Points to Consider on Human Somatic Cell Therapy and Gene Therapy, 1991;" “Guidelines for Human Somatic Therapy and Gene Therapy’, March, 1998”).
Since 1991 Dr. Epstein has studied immunity to influenza virus infection, including immunity to both viruses and vectors. Thus, her research has regulatory relevance to both vaccines and gene therapies. Her lab studies universal influenza vaccines based on conserved antigens that provide broad protection against influenza A viruses, including avian and swine-origin strains. The work in animals explores cross-protective immune mechanisms: systemic and mucosal antibodies and systemic and mucosal T cell responses. The World Health Organization has invited Dr. Epstein to discuss her research and to review the field of long-lasting, cross-protective influenza immunity and vaccines.
In addition to FDA and CBER scientific, regulatory policy, and management awards, she has received outside honors including invitations to give the Harry M. Rose Memorial Lecture in Infectious Diseases at Columbia University and the Distinguished Scientist Seminar at Emory University. She also received the AWIS Mentoring Award, and an honorary doctorate from the University of Toledo.
General Overview
Viruses and DNA vectors are used in two kinds of products regulated by CBER: vaccines and gene therapies. In this program, we study immune responses induced by vectors used to express proteins in the recipient: plasmid DNA (small rings of DNA derived from bacteria), adenovirus (a non-replicating version of a common virus that causes colds), and occasionally adeno-associated virus (AAV). These vectors are used in gene therapy vectors in some new vaccines. We need to understand immune responses to these vectors and the proteins they express, as well as assays to measure them, as a basis for sound regulatory decisions about preclinical animal models and clinical trials. In the case of gene therapy, pre-existing immunity to the vector used can cause adverse events and block effectiveness.
This program also addresses the Center- and Agency-wide public health priorities of controlling outbreaks of unexpected influenza strains, including pandemics. Influenza causes many serious illnesses and deaths each year in the US and globally. Current influenza vaccines are based on predominant strains of viruses circulating and infecting people, as determined by international surveillance. This is a time-consuming process with limited capacity, and strain predictions are uncertain. New strategies are needed to prepare for outbreaks of unexpected influenza virus strains, including avian influenza viruses such as H5N1 and pandemics such as the one in 2009. The goals are to reduce serious illness, hospitalization, and deaths, and to slow virus spread in the community.
This research program studies new approaches to influenza vaccination that protect regardless of which influenza virus strain emerges. We study experimental "one-size-fits-all" vaccines that could be available off the shelf. They could be rapidly deployed to help control an outbreak of an influenza virus against which available conventional vaccines do not protect. The new vaccines stimulate immune responses to viral proteins that are similar ("conserved") among all influenza A viruses. This type of protection is intended to reduce the severity of disease and the spread of infection during the delay until a strain-matched vaccine against the new influenza virus can be produced.
We immunize to conserved influenza proteins using plasmid DNA and adenoviruses. Experimental models include mice and also ferrets in which influenza virus can spread and cause disease similar to human influenza. We analyze immune responses of vaccinated animals, as well as protection against challenge infection. The experimental vaccines can protect against influenza infections lethal to unvaccinated animals for a broad range of virus strains, including lethal H5N1 (bird flu). With a single dose of vaccine given in the nose, we found protection as early as 2 weeks and as late as 10-12 months after vaccination. Although this kind of vaccine does not completely prevent virus from getting in, it reduces spread of infection to other animals placed in the same cages.
Scientific Overview
This lab studies immunity induced by viral and plasmid vectors and their expressed transgenes. Understanding immune responses is critically important to sound regulatory decisions about preclinical animal models and clinical trials, including immunological patient monitoring in IND studies. Regarding OTAT products, pre-existing immunity to vectors in gene therapy patients can cause adverse events and block effectiveness. OTAT also regulates various immunologically-based products, including some for control of viral infections. Additionally, control of seasonal and pandemic influenza is a Center- and Agency-wide public health priority in which OTAT participates, as is counter-bioterrorism defined as including pandemic influenza.
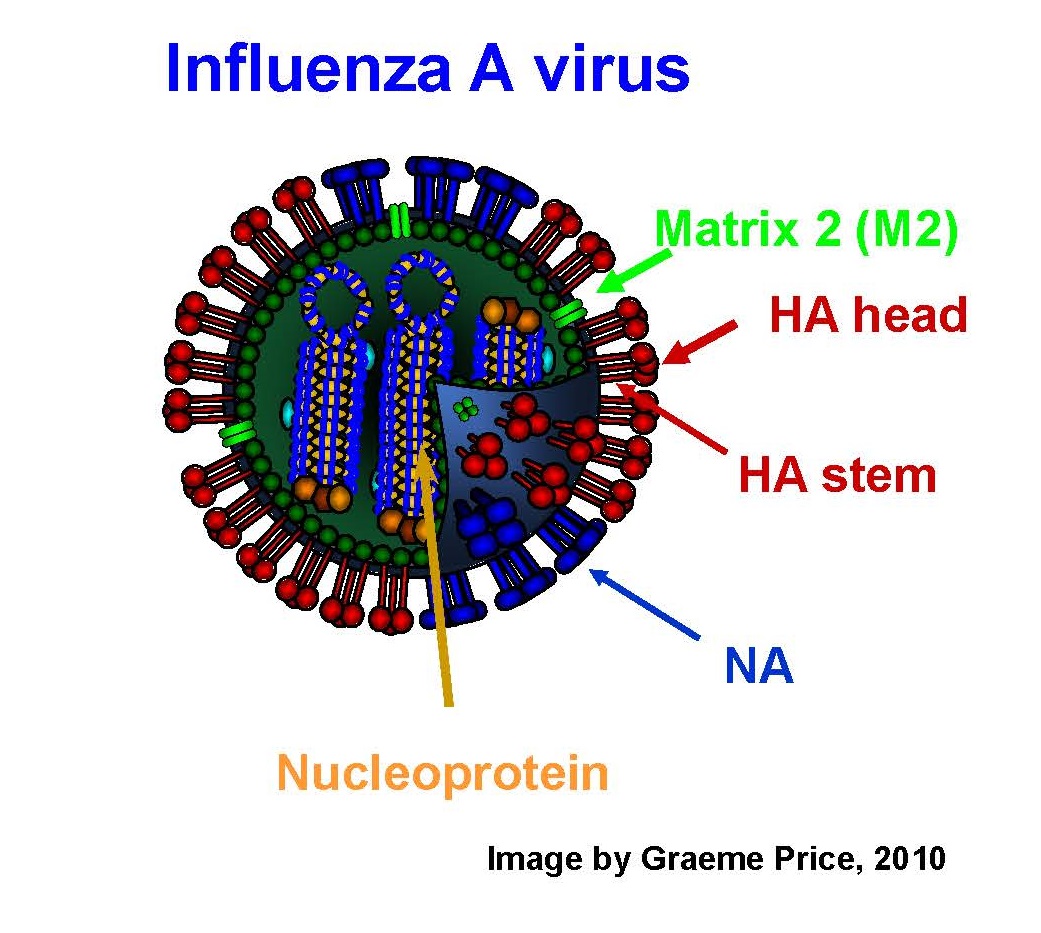
Influenza vaccination is made difficult by rapid evolution of the viral surface glycoproteins hemagglutinin and neuraminidase. However, broad cross-protection against influenza A (termed heterosubtypic immunity) is seen in various animal models: mice, ferrets, chickens, pigs, and cotton rats. It often permits low-grade infection, but reduces morbidity, mortality, and virus shedding. This lab studies universal vaccines based on conserved influenza antigens nucleoprotein (NP) and matrix (M). These vaccines provide broad cross-protection against diverse influenza A viruses of both groups 1 and 2. Thus, protection does not depend on predicting which strain or even subtype of influenza will circulate. These vaccines could fill the public health gap when conventional vaccines are not available, are insufficient in supply, or are a poor match for circulating virus.
We have compared various vaccination strategies in mice for potency of cross-protection. The candidates included cold-adapted (ca) influenza viruses, plasmid DNA, and recombinant adenoviruses (rAd) expressing conserved antigens A/NP, M2, or A/NP+M2. We use DNA prime-viral boost strategies, as well as rAd alone. The most potent cross-protection is seen with mucosal administration of rAd by the intranasal route, whether alone or as a boost after DNA priming. It results in survival and greatly reduced morbidity after challenge which is lethal to controls. The mucosal vaccinations give stronger IgA responses, greater and more long-lasting virus-specific activated T-cell responses in the lung, and better protection against weight loss following challenge. A single intranasal dose of A/NP+M2-rAd provided protection by 2 weeks and as late as10-12 months post-immunization against lethal challenge with H1N1 and H3N2 viruses, and highly pathogenic H5N1 viruses. We have also shown vaccine effectiveness in very old mice and in mice with previous exposure histories.
In another project, we study the effects of universal vaccination on transmission of influenza from infected donor mice to uninfected recipients. Intranasal vaccination of donors with A/NP+M2-rAd, which does not generate any neutralizing antibodies, reduced transmission to naive contacts. We have also studied vaccination in ferrets, an animal model with transmission and symptoms resembling human influenza. Ferrets are outbred and thus genetically diverse, like the human population. Prime-boost vaccination with either mucosal and parenteral rAd boosting, protected ferrets against lethal H5N1 challenge.
Occasionally this program has performed studies to investigate cross-protective immunity in humans: an archival study of records from the 1957 pandemic and a surveillance study during the 2009 pandemic to investigate cross-protective immunity.
Important Links
- Innovation and Regulatory Science- Research Summary: Universal influenza vaccine candidate reduces transmission of virus best when given nasally in mice
Publications
- PLoS One 2019 Apr 15;14(4):e0215321
The effect of respiratory viruses on immunogenicity and protection induced by a candidate universal influenza vaccine in mice.
Rowell J, Lo CY, Price GE, Misplon JA, Crim RL, Jayanti P, Beeler J, Epstein SL - Am J Epidemiol 2018 Dec 1;187(12):2603-14
Universal influenza vaccines: progress in achieving broad cross-protection in vivo.
Epstein SL - Vaccine 2018 Aug 6;36(32 Pt B):4910-8
Reduction of influenza virus transmission from mice immunized against conserved viral antigens is influenced by route of immunization and choice of vaccine antigen.
Price GE, Lo CY, Misplon JA, Epstein SL - Vaccine 2018 Feb 8;36(7):1008-15
Conventional influenza vaccines influence the performance of a universal influenza vaccine in mice.
Rowell J, Lo CY, Price GE, Misplon JA, Epstein SL, Garcia M - Open Forum Infect Dis 2017 Feb 12;4(2):ofx023
Surveillance study of influenza occurrence and immunity in a Wisconsin cohort during the 2009 pandemic.
Lo CY, Strobl SL, Dunham K, Wang W, Stewart L, Misplon JA, Garcia M, Gao J, Ozawa T, Price GE, Navidad J, Gradus S, Bhattacharyya S, Viboud C, Eichelberger MC, Weiss CD, Gorski J, Epstein SL - Science 2016 Nov 11;354(6313):706-7
First flu is forever.
Viboud C, Epstein SL - PLoS One 2016 Apr 7;11(4):e0153195
Age dependence of immunity induced by a candidate universal influenza vaccine in mice.
García M, Misplon JA, Price GE, Lo CY, Epstein SL - Gene Ther 2015 Oct;22(10):781-92
Enhanced T cell activation and differentiation in lymphocytes from transgenic mice expressing ubiquitination-resistant 2KR LAT molecules.
Rodriguez-Peña AB, Gomez-Rodriguez J, Kortum RL, Palmer DC, Yu Z, Guittard GC, Wohlfert EA, Silver PB, Misplon JA, Sommers CL, Feigenbaum L, Epstein SL, Caspi RR, Belkaid Y, Restifo NP, Samelson LE, Balagopalan L