Frequently Asked Questions (FAQs): Electronic Submissions Program for CBER-Regulated Products
Please feel free to contact esubprep@fda.hhs.gov for questions related to receipt of 3rd acknowledgements, technical rejection notices, test submissions, eCTD, eCTD waivers and FDA guidance resources.
Contents
- Why have I not received an eCTD 3rd acknowledgement email?
- What is a technical rejection notice?
- How do I submit my non-eCTD submission in Web Trader without it being broken up into multiple core ids?
- What is a single file submission?
- What file types are acceptable to submit for eCTD publishing?
- Where can I find eCTD guidance?
- How long does it take Electronic Submission Gateway (ESG) to deliver my submission to CBER?
- I have not received a 2nd acknowledgement, who do I contact?
- Why was my master file rejected?
- What should I do if I submitted to the wrong Center?
- My client has two or more application types under CBER review. Does CBER support cross application hyperlinks?
- Where should I place Video Files in eCTD?
- How do I request an eCTD waiver?
- What sequence number should I start my pre-submission or original application?
- Who do I contact for regulatory guidance?
1. Why have I not received an eCTD 3rd acknowledgement email?
Please provide a minimum of 2 hours before contacting esubprep@fda.hhs.gov. Causes for not receiving a 3rd acknowledgement include large volume of submissions in queue, failed eCTD validation or technical issues that prevents automated upload to CBER electronic repository. You will receive a technical rejection notice to your Web Trader or AS2 account.
More information regarding acknowledgement notifications can be found at https://www.fda.gov/industry/about-esg/esg-submission-process
2. What is a technical rejection notice?
CBER distributes technical rejection notices for eCTD and non-eCTD submissions. eCTD submissions that fail eCTD validation will receive a technical rejection.
Non-eCTD submissions may receive a technical rejection if we are not able to load to the electronic repository.
CBER technical rejection notices will provide the reason for rejection and, when possible, suggestions for correction. For additional information see Specifications for eCTD Validation Criteria and Providing Regulatory Submissions in Electronic Format — Receipt Dates.
Providing Regulatory Submissions in Electronic Format--Receipt Date | FDA
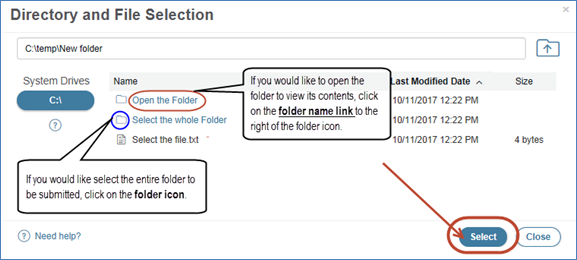
3. How do I submit my non-eCTD submission in Web Trader without it being broken up into multiple core ids?
In order to select a folder using Web Trader, you must click on the folder icon to the right of the folder name in the Web Trader Client. A single submission will only generate one core id number.
See item 17 of Web Trader FAQ.
4. What is a single file submission?
A single file submission is a non-eCTD submission, typically submitted in PDF. Only submissions not required to be submitted in eCTD can submit as a single file. See Providing Regulatory Submissions in Electronic Format - Using the eCTD Specifications - Certain Human Pharmaceutical Product Applications and Related Submissions Using the eCTD Specifications Guidance for Industry for submissions exempted from eCTD format.
5. What file types are acceptable to submit for eCTD publishing?
General Information Documents should be provided in PDF searchable format. For archival purposes, images and other document types should also be rendered into PDF format and retain searchable text.
Additional information related to PDF documents is available in the FDA technical specification FDA Portable Document Format (PDF) Specifications. Please review Specifications for File Format Types Using eCTD Specifications for information about other format types.
6. Where can I find eCTD guidance?
The Electronic Common Technical Document (eCTD) webpage and eCTD Resources webpage is a good starting point to identify eCTD related guidance and resources.
7. How long does it take Electronic Submission Gateway (ESG) to deliver my submission to CBER?
The webpage ESG Submission Times provides estimated transaction times for the upload, delivery, and acknowledgements for submissions.
8. I have not received a 2nd acknowledgement, who do I contact?
Contact ESGhelpdesk@fda.hhs.gov to get the routing status of your submission.
9. Why was my master file rejected?
Master Files (MFs), except Type III MFs, are required to be submitted to the Agency electronically in eCTD format. This requirement can be found in FDA’s Providing Regulatory Submissions in Electronic Format - Certain Human Pharmaceutical Product Applications and Related Submissions Using the eCTD Specifications Guidance for Industry.
For additional information please see Drug Master Files Guidance for Industry.
10. What should I do if I submitted to the wrong Center?
Submissions Tracking Numbers (STN) not supported by CBER will fail electronic validation. eCTD submissions submitted to the wrong Center will receive a technical rejection notice via ESG from CBER. Non eCTD submissions will be handled on a case-by-case basis.
11. My client has two or more application types under CBER review. Does CBER support cross application hyperlinks?
CBER does not support cross-application hyperlinks. Hyperlinks to sections within a document are acceptable.
12. Where should I place Video Files in eCTD?
In accordance with the Specifications for File Format Types Using eCTD Specifications, video files should only be submitted in eCTD section “1.15 Promotional material” in support of Promotional Labeling and Advertising Material submissions.
13. How do I request an eCTD waiver?
Waivers from submitting in eCTD can only be requested for certain Positron Emission Tomography (PET) and certain Type II Drug Master File submissions. Please review section III of the guidance Providing Regulatory Submissions in Electronic Format-Certain Human Pharmaceutical Product Applications and Related Submissions Using the eCTD Specifications, for information about types of waivers, how and where to submit.
14. What sequence number should I start my pre-submission or original application?
Original applications and pre-submissions should start with sequence number 0001.
For questions about ESG or Web Trader contact ESGhelpdesk@fda.hhs.gov
15. Who do I contact for regulatory guidance?
- Contact your CBER Regulatory Project Manager (RPM) or review office that reviews type of product you are submitting.
- Contact cberocod@fda.hhs.gov for consumer questions.
- Contact Industry.Biologics@fda.hhs.gov for questions about DMF, manufacturing, technical training, and general regulatory questions.
- Contact CBER-edata@fda.hhs.gov for questions about submitting study data
- Contact cber-reg-ops-device@fda.hhs.gov for questions about device submissions including 510K, IDE and PMA.