Drug Trials Snapshot: TAVNEOS
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the TAVNEOS Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
TAVNEOS (avacopan)
(tav' nee ose)
ChemoCentryx, Inc.
Original Approval date: October 7, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TAVNEOS is a drug used with other medicines (such as glucocorticoids) to treat adults with severe active anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (specifically, granulomatosis with polyangiitis [GPA], formerly known as Wegener’s granulomatosis, and microscopic polyangiitis [MPA]).
Vasculitis is a group of diseases that causes inflammation of blood vessels that then results in poor blood flow. This inflammation can lead to organ and tissue damage throughout the body, including the lungs and kidney.
How is this drug used?
TAVNEOS is a capsule taken by mouth. Three capsules are taken two times a day (morning and evening) with food.
Who participated in the clinical trials?
The FDA approved TAVNEOS based on evidence from a clinical trial of 330 patients with severe active ANCA-associated vasculitis (GPA or MPA). The trial was conducted at 143 sites in 18 countries including the United States. This trial assessed both efficacy and safety.
What are the benefits of this drug?
In the clinical trial, a greater proportion of patients who received TAVNEOS for one year with other medicines (including glucocorticoids) achieved sustained disease remission compared to patients who received other medicines without TAVNEOS. The proportion of patients who achieved remission after 6 months of treatment was similar.
What are the benefits of this drug (results of trials used to assess efficacy)?
The primary endpoint was remission at Week 26 and sustained remission at Week 52.
Table 1. Sustained Remission at Week 52 in Phase 3 Trial (Intent-to-Treat Population)
|
|
Prednisone |
TAVNEOS N=166 |
Estimate of |
|
|---|---|---|---|---|
|
Sustained remission at Week 52 |
90 (54.9%) |
109 (65.7%) |
12.5%b |
0.013 |
|
95% CI |
46.9, 62.6c |
57.9, 72.8c |
2.6, 22.3d |
Source: TAVNEOS Prescribing Information
a 2-sided p-value of Summary Score Test (Agresti 2013)
b Summary Score estimate of the common difference in remission rates (Agresti 2013) by using inverse-variance stratum weights
c Clopper and Pearson exact CI
d Miettinen-Nurminen (Score) confidence limits for the common difference in remission rates
Abbreviations: CI, confidence interval; N, number of patients in the analysis population for the specified treatment group; n, number of patients with disease remission; % = 100*n/N
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: TAVNEOS worked similarly in male and female patients.
- Race: TAVNEOS worked similarly in White and Asian patients. The number of patients in other race categories was small; therefore, differences in how TAVENOS worked in other races should be interpreted with caution.
- Age: TAVNEOS worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
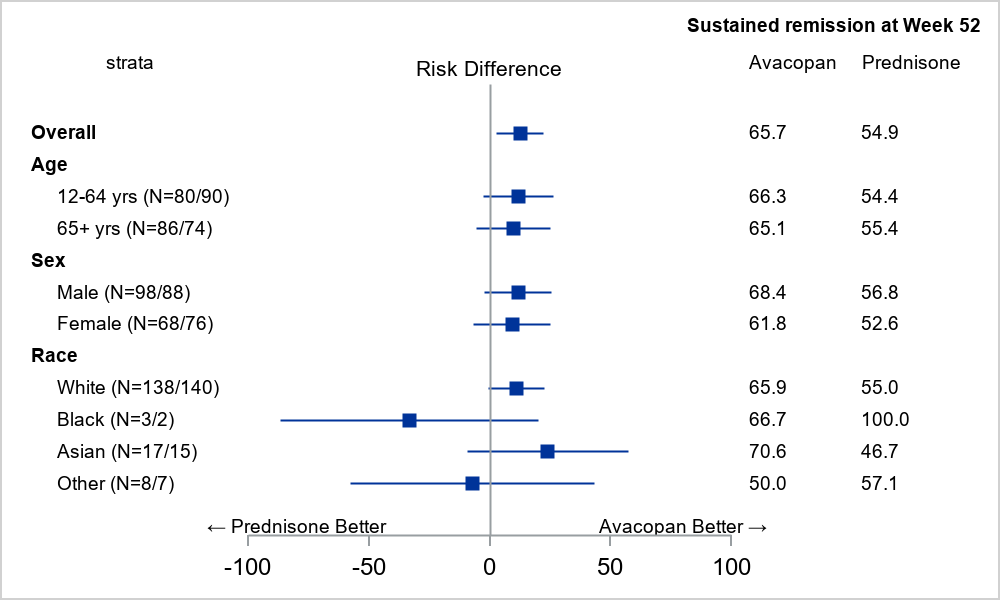
The efficacy results by demographic subgroups were consistent with the results of the overall population. Figure 1 below shows sustained remission at Week 52 by demographic subgroups of age, sex, and race. Similar results were seen by demographic subgroups at Week 26.
Figure 1. Effects of TAVNEOS on Sustained Remission at Week 52 by Demographic Subgroups of Age, Sex, and Race
Source: Adapted from FDA Review
Point estimate and 95% confidence interval using normal approximation.
The notation N = XX/YY indicates the sample size for avacopan (TAVNEOS) and prednisone, respectively.
What are the possible side effects?
TAVNEOS may cause serious side effects including liver problems, serious allergic reactions, hepatitis B virus (HBV) reactivation, and serious infections.
The most common side effects that occurred in the TAVNEOS group are pneumonia, granulomatosis with polyangiitis (GPA), acute kidney injury, and urinary tract infection.
What are the possible side effects (results of trials used to assess safety)?
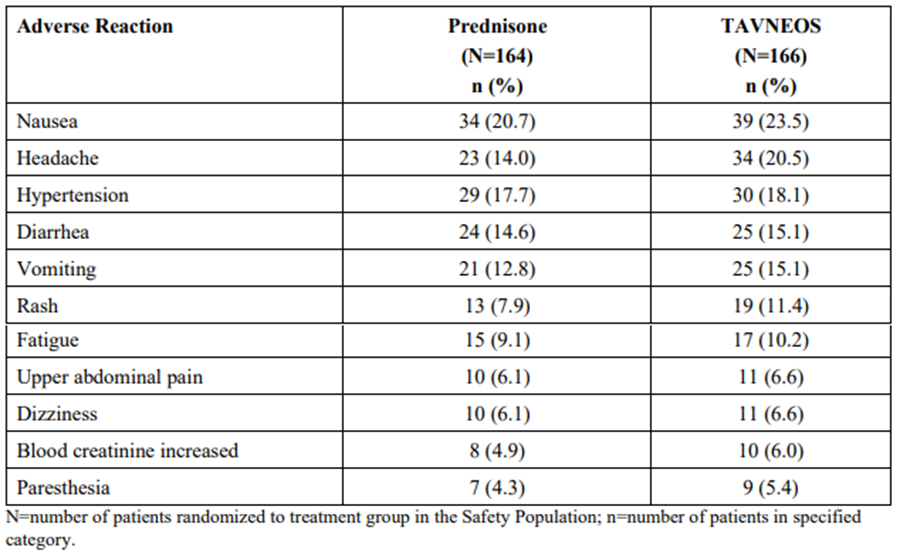
Table 2 is a summary of the most common adverse reactions observed in patients treated with TAVNEOS at the recommended dose that exceeded placebo in the clinical trial.
Table 2. Adverse Reactions* Reported in ≥5% of Patients and Higher in the TAVNEOS Groups as Compared With the Prednisone Group in the Phase 3 Trial
Source: TAVNEOS Prescribing Information
* Hepatic-related adverse events (including hepatobiliary adverse reactions and liver enzymes abnormalities) occurred in 22 patients (13.3%) in the TAVNEOS group and 19 patients (11.6%) in the prednisone group.
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: The occurrence of side effects was similar in male and female patients.
- Race: Generally, the occurrence of side effects was similar in White and Asian patients. The number of patients in other race categories was small; therefore, differences in the occurrence of side effects in other races should be interpreted with caution.
- Age: The occurrence of serious side effects was greater in patients older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 3 below summarizes the occurrence of adverse events by sex, race, and age subgroups.
Table 3. Subgroup Analysis of Treatment Emergent Adverse Events (Overall and Serious) by Sex, Race, and Age
|
|
TEAE |
Serious TEAE |
||
|---|---|---|---|---|
|
Prednisone |
TAVNEOS |
Prednisone |
TAVNEOS |
|
|
Sex |
||||
|
Male |
87/88 (98.9) |
97/98 (99.0) |
43/88 (48.9) |
43/98 (43.9) |
|
Female |
74/76 (97.4) |
67/68 (98.5) |
31/76 (40.8) |
27/68 (39.7) |
|
Race |
||||
|
White |
137/140 (97.9) |
136/138 (98.6) |
64/140 (45.7) |
56/138 (40.6) |
|
Asian |
15/15 (100) |
17/17 (100) |
7/15 (46.7) |
7/17 (41.2) |
|
Black or African American |
2/2 (100) |
3/3 (100) |
0/2 (0) |
2/3 (66.7) |
|
Others |
7/7 (100) |
8/8 (100) |
3/7 (42.9) |
5/8 (62.5) |
|
Age, years |
||||
|
<65 |
88/90 (97.8) |
79/80 (98.8) |
38/90 (42.2) |
28/80 (35.0) |
|
≥65 |
73/74 (98.6) |
85/86 (98.8) |
36/74 (48.6) |
42/86 (48.8) |
Source: Adapted from FDA Review
Abbreviations: TEAE, treatment-emergent adverse event
DEMOGRAPHICS SNAPSHOT
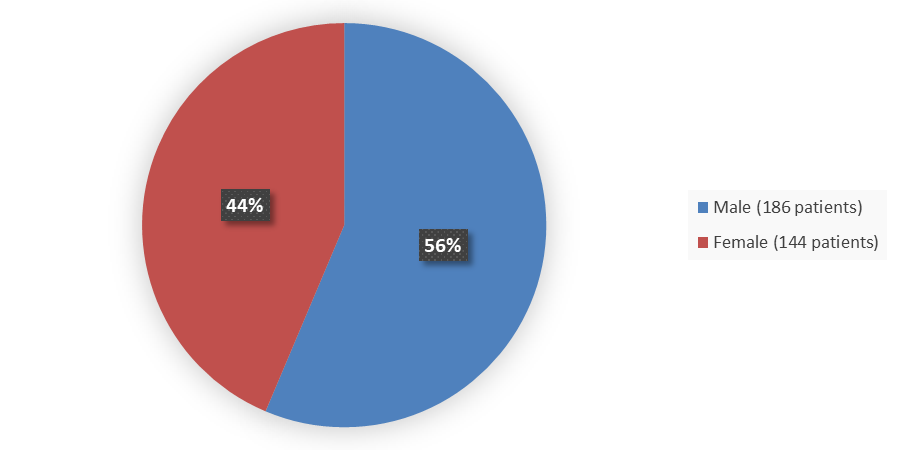
Figure 2 summarizes how many male and female patients were enrolled in the clinical trial used to evaluate the efficacy and safety of TAVNEOS.
Figure 2. Baseline Demographics by Sex
Source: Adapted from FDA Review
Figure 3 summarizes the percentage of patients by race enrolled in the clinical trial used to evaluate the efficacy and safety of TAVNEOS.
Figure 3. Baseline Demographics by Race
Source: Adapted from FDA Review
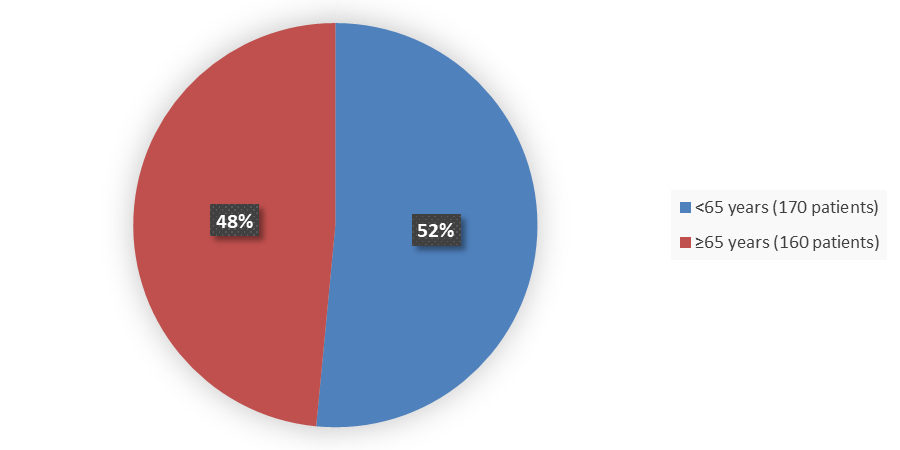
Figure 4 summarizes the percentage of patients by age enrolled in the clinical trial used to evaluate the efficacy and safety of TAVNEOS.
Figure 4. Baseline Demographics by Age
Source: Adapted from FDA Review
Who participated in the trials?
Table 4 below summarizes demographics of patients in the clinical trial.
Table 4. Baseline Demographics of Patient Population in Clinical Trial
|
|
Prednisone |
TAVNEOS |
|---|---|---|
|
Sex, n (%) |
||
|
Male |
88 (53.7) |
98 (59.0) |
|
Female |
76 (46.3) |
68 (41.0) |
|
Age, years |
||
|
Mean (SD) |
60.5 (14.5) |
61.2 (14.6) |
|
Min, max |
15, 88 |
13, 83 |
|
Race, n (%) |
||
|
White |
140 (85.4) |
138 (83.1) |
|
Black or African American |
2 (1.2) |
3 (1.8) |
|
Asian |
15 (9.1) |
17 (10.2) |
|
Other |
7 (4.3) |
8 (4.8) |
|
Ethnicity, n (%) |
||
|
Hispanic or Latino |
5 (3.0) |
7 (4.2) |
|
Not Hispanic or Latino |
157 (95.7) |
151 (91.0) |
|
Not reported/Unknown |
2 (1.2) |
8 (4.8) |
|
Region, n (%) |
||
|
North America |
25 (15.2) |
34 (20.5) |
|
Europe and rest of world except Japan |
129 (78.7) |
121 (72.9) |
|
Japan |
10 (6.1) |
11 (6.6) |
Source: Adapted from FDA Review
How were the trials designed?
TAVNEOS was evaluated in one pivotal clinical trial of 330 patients with severe active ANCA-associated vasculitis.
In the pivotal clinical trial, patients were randomly assigned to receive TAVNEOS or placebo for 52 weeks. Patients in the placebo group received a glucocorticoid taper over 20 weeks. Neither the patients nor healthcare providers knew which medication was being given. Patients in both groups received background immunosuppressive treatment (cyclophosphamide or rituximab) and were allowed to receive additional glucocorticoids. The benefit of TAVNEOS in comparison to placebo was assessed by proportion of patients who achieved remission at Week 26 and sustained remission at Week 52. Data from this trial were also analyzed for the assessment of side effects.
How were the trials designed?
TAVNEOS was evaluated in a double-blind, active-controlled study in 330 patients with newly diagnosed or relapsed ANCA-associated vasculitis. Patients were randomized 1:1 to one of the following treatment groups:
- TAVNEOS group (N=166): Patients received TAVNEOS 30 mg twice daily for 52 weeks plus prednisone-matching placebo for 20 weeks
- Prednisone group (N=164): Patients received TAVNEOS-matched placebo twice daily for 52 weeks plus prednisone (tapered from 60 mg/day to 0 mg/day over 20 weeks)
All patients in both groups received one of the following standard immunosuppressive regimens:
- Cyclophosphamide, intravenous (IV): 15 mg/kg IV up to 1.2 g maximum every 2 to 3 weeks for 13 weeks, followed by oral azathioprine 1 mg/kg/day with titration up to 2 mg/kg/day (or mycophenolate mofetil at a target dose of 2 g/day if azathioprine was contraindicated) from Week 15 onwards
- Cyclophosphamide, oral: 2 mg/kg/day (maximum 200 mg/day) for 14 weeks, followed by azathioprine 1 mg/kg day with titration up to 2 mg/kg/day (or mycophenolate mofetil at a target dose of 2 g/day if azathioprine was contraindicated) from Week 15 onwards
- Rituximab, IV: 375 mg/m2 once weekly for 4 weeks without azathioprine or mycophenolate mofetil
Glucocorticoids were allowed as pre-medication for rituximab to reduce hypersensitivity reactions, taper after glucocorticoids given during the Screening period, treatment of persistent vasculitis, worsening of vasculitis, or relapses, as well as for non-vasculitis reasons such as adrenal insufficiency.
The primary endpoints of the study were disease remission at Week 26 and sustained disease remission at Week 52. Disease remission was defined as achieving a Birmingham Vasculitis Activity Score (BVAS) of 0 and no use of glucocorticoids for treatment of ANCA-associated vasculitis from Week 22 to Week 26. Sustained remission was defined as remission at Week 26 and remission at Week 52, without relapse between Week 26 and Week 52. Remission at Week 52 was defined as BVAS of 0 and no use of glucocorticoids for treatment of ANCA-associated vasculitis from Week 48 to Week 52. Relapse was defined as occurrence of one major item, at least three non-major items, or one to two non-major items for at least two consecutive visits on the BVAS after remission (BVAS of 0) had been achieved.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.