Bispecific Antibodies: An Area of Research and Clinical Applications
Bispecific antibodies (BsAbs) have two distinct binding domains that can bind to two antigens or two epitopes (an antigen part) of the same antigen simultaneously. Over the past two decades, BsAb development has been revolutionized due to genetic engineering approaches that enable a wide variety of molecular structures that offer different advantages and disadvantages.
BsAbs are next generation monoclonal antibodies (mAbs). Typical mAbs target one epitope.1 FDA approved the first mAb, Orthoclone, in 1986 to help prevent rejection in organ transplantation.2 The agency has now approved well over 100 novel mAbs3 for cancer, autoimmune and infectious diseases, and inflammatory conditions, among other indications.4
BsAbs have joined mAbs on the therapeutic antibody stage. By targeting two antigens or epitopes, they can cause multiple physiological or anti-tumor responses, which may be independent or connected. These treatments may act like a “cocktail” of two mAbs, but drug developers need to manufacture only one molecule and patients may need only one antibody treatment. Plus, their synergistic (or cooperative) features may produce more significant treatment effects.
Right now, most BsAbs in development aim to treat cancer, but others are focused on chronic inflammatory, autoimmune, and neurodegenerative diseases; vascular (the body’s network of blood vessels), ocular (eye-related), and hematologic (blood-related) disorders; and infections.
Research and development efforts are leading to clinical applications. There are over 100 BsAbs in clinical development, most in the early stages.5 Since 2014, FDA has approved nine BsAb marketing applications to treat cancer, as well as hematologic and ocular diseases. (See chart below). In the future, FDA anticipates there will be a spectrum of BsAbs developed to prevent, treat, or diagnose diseases.1
The agency has been encouraging drug development in this area. In 2021, FDA finalized a guidance on BsAb development programs,1 which discusses aspects for chemistry, manufacturing, and controls (CMCs) as well as nonclinical and clinical development programs. It also describes the challenges, such as immunogenicity (or causing an immune response) related to the novel structures. In addition, the guidance1 recommends types of data to support BsAb approvals.
FDA staff have been conducting research to analyze different BsAb molecular formats using physiochemical and biological approaches. The goals of these studies are not to develop drugs, but rather to understand how different formats of BsAbs impact quality aspects (e.g., product characterization, bioassay or potency assay development, and stability).
BsAbs in Triple-Negative Breast Cancer
Multiple Methods of Action
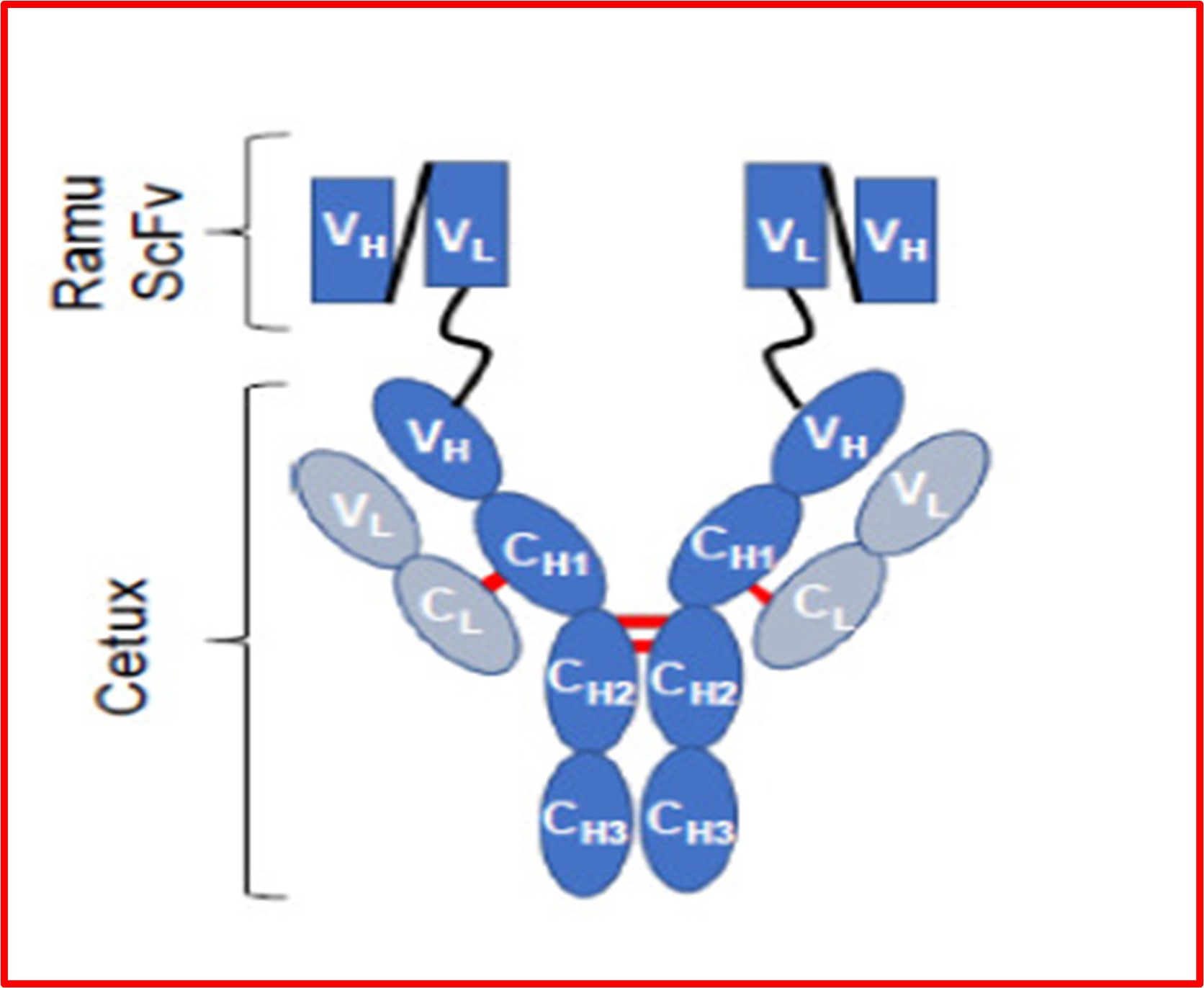
The majority of BsAbs to-date target different types of cancer. Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor 2 (VEGFR2) proteins are already validated targets and work together through complex cell signaling pathways to spur tumor growth. Using triple-negative breast cancer as a model, researchers6 generated a BsAb and examined if it inhibits tumor growth via multiple mechanisms of action using cellular and xenograft mouse models (models of mice with tumors implanted into them). The BsAb had a full-length mAb antibody (cetuximab immunoglobin G or IgG) that looked like the letter Y, with two additional mAb fragments (ramucirumab) fanning out from the top of the Y’s arms. The full-length antibody was targeting the EGFR protein and the fragments were targeting VEGFR2.
Investigators found that the BsAb spurred anti-tumor activity in several ways, namely by stopping cancer cell growth and disrupting the cell signaling pathways (the chatter that encourages tumor growth). These results help scientists understand the complicated mechanisms of action and develop bioassays for BsAbs. In the animal model, the mice receiving the BsAb had significantly slower tumor growth compared to mice treated with saline or a mAb that targeted only EGFR or VEGFR2. Mice also did not lose weight during treatment, suggesting that the BsAb did not cause significant systemic (full body) side effects.
These research findings show that a BsAb that targets EGFR and VEGFR2 can stop tumor growth through multiple mechanisms of action and merits further investigation as a treatment for triple-negative breast cancer.
Format Matters
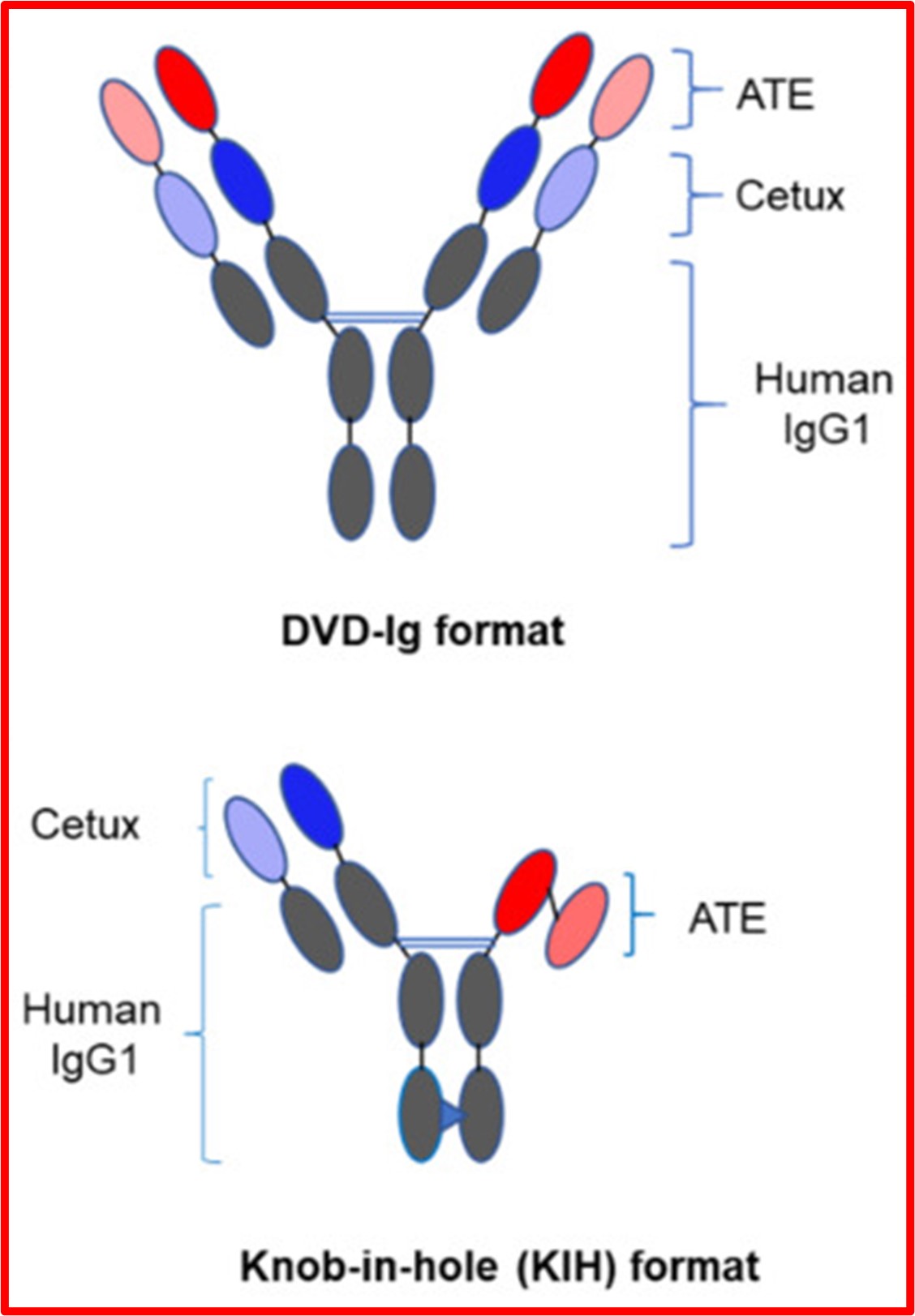
In another triple-negative breast cancer study, researchers examined the BsAb molecular formats using physicochemical and biological approaches. This study focused on two formats that are commonly used in clinical settings: the dual-variable domain immunoglobin (DVD-Ig) with two binding sites against each antigen, and the “knob-in-hole” (KIH) with one binding site against each antigen. In this study, the antibodies were targeting the same antigens, EGFR and PD-L1.
Both BsAbs had a Y shape; however, for the KIH, investigators created a “knob” on one side of the Y stem to fit into a “hole” on the other side to ensure a correct pairing, almost like puzzle pieces.
Researchers compared key quality attributes and biological activities of BsAbs with different molecular formats and analyzed how the antibodies stopped tumor growth in the cellular and animal models. They found that although both therapies bound to the antigen targets, the DVD-Ig had a slightly stronger binding affinity than the KIH, according to one binding test. Similarly, DVD-Ig was slightly stronger in its antitumor activity. Researchers attributed this difference to flexibility of the DVD-Ig molecule and the DVD-Ig’s ability to bind to two molecules of each antigen simultaneously.
The study found that the type of cells used for analysis (MDA-MB-231 cells and BT-20 cells) and the assays to detect antitumor activities affected detection capabilities. For instance, one assay (cell viability) could detect antitumor effects in both cell lines, but another (trypan blue cell proliferation) was not sensitive enough to detect antitumor effects in the BT-20 cells. When developing methods to control the potency of BsAbs, it is important to understand which cell lines and assays are optimal. This knowledge can help reviewers analyze and assess BsAb quality.
BsAbs for COVID-19
Early in the pandemic, mAbs targeting the SARS-CoV-2 viral spike protein showed promise as treatments for SARS-CoV-2 infection and FDA authorized several therapies for certain patients with mild-to-moderate COVID-19. However, monoclonal antibody therapies that the agency previously made available under Emergency Use Authorization are not currently authorized in the U.S. until further agency notice. This is a result of the rapid emergence and high prevalence in the U.S. of SARS-CoV-2 variants that contain amino acid changes in the epitopes within the spike protein that the monoclonal antibodies target. Such changes in the virus have greatly reduced the activity of these monoclonal antibody therapies.
In response to this challenge, researchers have shifted their focus to developing BsAbs to simultaneously target two epitopes on the virus's spike protein. This approach increases the likelihood of maintaining binding and neutralizing activities against a variety of virus strains, including those that have undergone mutations. By engaging with two epitopes, BsAbs have the potential to overcome the limitations imposed by viral evolution, ensuring a broader spectrum of neutralization against emerging variants. CDER scientists have developed potency assays to comprehensively evaluate these products.
Generally, investigators found antibodies that attached well also neutralized the virus to a greater extent. The two assays that researchers created can help assess future BsAbs developed to treat the SARS-CoV-2 variants.
Moving Forward
We can expect to see more research, development, and clinical applications of BsAbs. But the field is not stopping here.
Multispecific antibodies, which can target multiple antigens simultaneously, are also moving forward. There is a dizzying array of potential formats for multispecific antibodies, which may lead to treatments for diseases with no or few therapies. FDA will continue to help move the needle in this therapeutic area. The agency’s guidance1 on BsAbs notes the principles in that publication may inform the development of multispecific products as well. FDA will also continue to review therapeutic antibody drug applications, expeditiously if appropriate; answer questions; and conduct further research.
FDA-Approved Bispecific Antibodies
| Trade Name | Active Ingredient | Year Approved | Indication |
|---|---|---|---|
| Blincyto | blinatumomab | 2014 | To treat Philadelphia chromosome-negative relapsed or refractory B cell precursor acute lymphoblastic leukemia |
| Hemlibra | emacizumab-kxwh | 2017 | To prevent or reduce the frequency of bleeding episodes in hemophilia A with factor VIII inhibitors |
| Rybrevant | amivantamab-vmjw | 2021 | To treat locally advanced or metastatic non-small cell lung cancer with certain mutations |
| Kimmtrak* | tebentafusp-tebn | 2022 | To treat a form of unresectable or metastatic uveal melanoma |
| Vabysmo | faricimab-svoa | 2022 | To treat neovascular (wet) age-related macular degenerated and diabetic macular edema |
| Tecvayli | teclistamab-cqyv | 2022 | To treat relapsed or refractory multiple myeloma |
| Lunsumio | mosunetuzumab-axgb | 2022 | To treat relapsed or refractory follicular lymphoma |
| Epkinly | epcoritamab-bysp | 2023 | To treat relapsed or refractory diffuse large B-cell lymphoma |
| Columvi | glofitamab-gxbm | 2023 | To treat relapsed or refractory diffuse large B-cell lymphoma or large B-cell lymphoma |
*Kimmtrak is technically a bispecific molecule, not a bispecific antibody. Like some of the other bispecific antibodies used to treat some cancers, Kimmtrak has one arm using an antibody fragment to bring killer T cells to the tumor. Kimmtrak’s other arm is an analogous structure found on T cells, the T cell receptor, instead of an antibody fragment to target a tumor antigen.
1US Food and Drug Administration. FDA guidance: Bispecific antibody development programs. 2021 May. Accessed May 18, 2023.
2Lu RM, Hwang, YC, Liu IJ. Development of therapeutic antibodies for the treatment of diseases. Journal of Biomedical Science. 2020 Jan. 27, 1. https://doi.org/10.1186/s12929-019-0592-z
3Mullard A. FDA approves 100th monoclonal antibody product. Nature. 2021 July 491-495.
4Ecker, DM, SD Jones, and HL Levine. The Therapeutic Monoclonal Antibody Market, mAbs, 2015. 7(1):9–14, doi: 10.4161/19420862.2015.989042.
5Brinkmann U, Kontermann RE. Bispecific antibodies. Science. 2021 May. 916-917.
6Mohan N, Luo X, Shen Y et al. A novel bispecific antibody targeting EGFR and VEGFR2 is effective against triple-negative breast cancer via multiple mechanisms of action. Cancers. 2021 Mar. 13(5) 1027.
7Mohan N, Agrawal A, Shen Y et al. Comparative characterization of different molecular formats of bispecific antibodies targeting EGFR and PD-L1. Pharmaceutics 2022 June. 14(7).
8Dean A, Stauft C, Twomey J et al. Comparative assessment of the binding and neutralization activity of bispecific antibodies against SARS-CoV-2 variants. Antib Ther. 2022 Dec 29;6(1):49-58.