Other Professional Resources

Project Facilitate
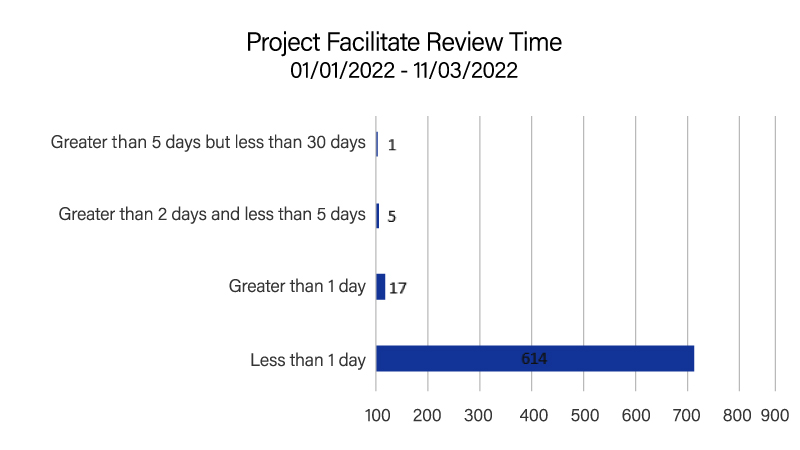
Project Facilitate is a comprehensive program assisting oncology healthcare and regulatory professionals in submitting Expanded Access requests to the FDA. In addition to providing support through email and phone, Project Facilitate staff also review all CDER oncology single-patient expanded access applications.
In 2022, Project Facilitate handled 397 phone calls and 637 expanded access applications; 96% (614/637) of applications were reviewed within 24 hours. This is consistent with the Project Facilitate mission to improve efficiency of review of oncology Expanded Access requests. The Project facilitate staff continued their educational outreach through presentations to clinics and hospital systems as well as an abstract submitted to the 2022 American Society of Clinical Oncology Annual Meeting.
Oncology Guidances
The OCE led the development of five new draft guidances and 10 final guidances for oncology drug development in 2022. See all current oncology guidance documents at OCE Guidance Documents.
Draft Guidances:
- Tissue Agnostic Drug Development in Oncology, October 2022
- Characterizing, Collecting, and Reporting Immune-Mediated Adverse Reactions in Cancer Immunotherapeutic Clinical Trials, October 2022
- Real-Time Oncology Review, July 2022
- Use of Circulating Tumor Deoxyribonucleic Acid for Early-Stage Solid Tumor Drug Development, May 2022
- Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Subgroups in Clinical Trials, April 2022
Final Guidances:
- Pharmacokinetic-Based Criteria for Supporting Alternative Dosing Regimens of Programmed Cell Death Receptor-1 (PD-1) or Programmed Cell Death-Ligand 1 (PD-L1) Blocking Antibodies for Treatment of Patients with Cancer, December 2022
- Cross Labeling Oncology Drugs in Combination Regimens, November 2022
- Acute Myeloid Leukemia: Developing Drugs and Biological Products for Treatment, October 2022
- Cancer Clinical Trial Eligibility Criteria: Available Therapy in Non-Curative Settings, July 2022
- Renal Cell Carcinoma: Developing Drugs and Biologics for Adjuvant Treatment, June 2022
- Bladder Cancer: Developing Drugs and Biologics for Adjuvant Treatment, June 2022
- Advanced Prostate Cancer: Developing Gonadotropin-Releasing Hormone Analogues, May 2022
- Inclusion of Older Adults in Cancer Clinical Trials, March 2022
- Expansion Cohorts: Use in First-In-Human Clinical Trials to Expedite Development of Oncology Drugs and Biologics Guidance for Industry, March 2022
- Master Protocols: Efficient Clinical Trial Design Strategies to Expedite Development of Oncology, March 2022
OCE Publications and Approval Announcements
The OCE’s strong support for publications by FDA oncology/hematology staff continued in 2022, resulting in 87 articles in scientific journals.
OCE’s Communications Team works with FDA clinical reviewers to issue approval announcements for most oncology/malignant hematology approvals; 40 of these were posted in 2022.