Thousands of Sites, Less Than 100 Investigators: New Authorities Help FDA Maintain Oversight of Clinical Research
By: Chrissy J. Cochran, PhD, Director, Office of Bioresearch Monitoring Operations
To protect and promote the public health is the mission of the U.S. Food and Drug Administration (FDA). Evidence of our public health protection is seen every day through recalls of violative products, warning letters issued to violators of our regulations, and screening of imported products from around the world at our nation’s ports of entry. What may be less obvious to some is how FDA promotes public health. We promote public health through communication and consumer education, but our primary means is by assessing medical innovations for safety and effectiveness prior to marketing in the U.S. It is a responsibility we take very seriously, and we apply the utmost scientific rigor to ensure we are achieving our mission on behalf of U.S. patients.
Clinical trials are an integral part of new product discovery and development and are required by the FDA before a new drug, biological product, or medical device can be approved for marketing. Reliable and accurate data from clinical trials is the cornerstone of FDA’s evaluation of a new medical product. FDA’s review of clinical trial data informs the agency’s decisions about the suitability of the medical product under review. Historically this research has been preceded by laboratory and animal testing to determine potential toxicity and other safety concerns. New methods, such as computer modeling and so-called “organ chips” are under development by the private sector to reduce or eliminate testing on research animals and potentially accelerate this component of medical product development in the coming years.
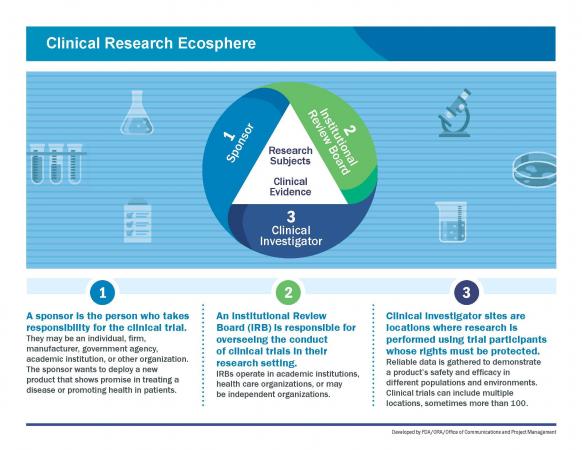
The clinical research ecosphere has three elements. The FDA expects sponsors, clinical investigators, and Institutional Review Boards (IRBs) to follow the regulations regarding participants in clinical trials and inspects all three parts of the “Research Triangle” to ensure compliance and identify data irregularities.
The FDA’s bioresearch monitoring (BIMO) program is made up of a comprehensive set of onsite inspections and data audits monitoring all aspects of the conduct and reporting of FDA-regulated research. The Office of Regulatory Affairs (ORA), Office of Bioresearch Monitoring Operations (OBIMO) takes the lead role in conducting inspections of clinical trial sites, IRBs, and sponsors. We conduct for-cause and surveillance inspections of clinical and bioavailability test sites to determine whether the site’s study conduct assures the integrity and reliability of study data. Inspection findings are utilized by the FDA’s centers during the review of their medical product applications. Although the program does not have the resources to inspect every clinical trial site; a representative sample is inspected to ensure that requirements regarding trial participants, study protocols, data collection activities, and other elements of clinical research are conducted appropriately. Data analytics are applied to ensure the agency assesses the most critical sites’ performance and compliance, and to determine whether study results are reliable across the spectrum of clinical research.
During the pandemic, OBIMO was on the front lines conducting inspections of COVID vaccines and therapies, and other potentially life-saving medical products, to ensure the data could be relied upon by center reviewers. The FDA also worked to enhance collaboration with foreign regulatory counterparts to share information, increase efficiency in reviews, and optimize global inspection resources. Our staff collaborates with international partners to effectively assess the scope and significance of any misconduct identified during inspections or the review of a marketing application.
Compliance and Enforcement Activities
During inspections, FDA investigators verbally discuss any potential violations they observe, communicating them to the most responsible individual. That individual may respond verbally to the investigator or in writing following the inspection. Responses are considered when determining the impact of violations on the reliability of the data submitted.
The FDA has regulatory and criminal authority over sponsors and clinical investigators. Firms that engage in illegal clinical research activity, including knowingly enrolling subjects failing to meet eligibility criteria, falsifying laboratory results, falsifying medical records, and falsely representing subjects as taking the drug being studied when they were not, are referred to FDA’s Office of Criminal Investigations (OCI). The OCI has brought criminal charges against multiple groups of individuals and organizations over the last several years. When FDA finds falsification at a clinical research site, FDA can disqualify the clinical investigator from conducting FDA-regulated research. Falsification of data is a criminal act, and the agency works with the U.S. Department of Justice to bring criminals to justice.
Looking forward, the Food and Drug Omnibus Reform Act (FDORA) was enacted December 2022, providing FDA’s BIMO program with new authority to request records in advance of or in lieu of BIMO inspections by amending the Food, Drug and Cosmetic Act section 704(a)(4). Further, Section 704 of the Act was also amended to clearly articulate FDA’s authority to enter and inspect all establishments engaged in activities conducting research in support of marketing applications for FDA-regulated products. FDA is implementing these new authorities to further enhance oversight of clinical trials.