Assessing the Impact of Opioid Overdose and Opioid Antagonist Dosing to Support Medical Product Development
CDER researchers have developed a comprehensive translational model to evaluate dosing strategies for using opioid receptor antagonists to treat opioid overdose.
Background
Overdoses of opioid drugs can cause death by respiratory depression, in which a person’s breathing becomes slower and shallower and the lungs fail to exchange carbon dioxide and oxygen efficiently. The Centers for Disease Control and Prevention report that deaths in the US from opioid overdose in 2020 exceeded 60,000,1 and there are additional concerns that synthetic opioids (such as fentanyl), given their potency and accessibility, could be used as chemical weapons.2 Therefore, there have been increased efforts to develop and make available products to treat respiratory depression in community health settings.
Naloxone is an opioid antagonist indicated for the emergency treatment of known or suspected opioid overdose as manifested by respiratory and/or central nervous system depression. It works by binding to opioid receptors in the central nervous system and blocking the effects of other opioids, such as heroin, morphine, oxycodone, and synthetic opioids like fentanyl. FDA has recently approved naloxone products that can be administered as injections or intranasally by individuals without extensive medical training to help prevent opioid overdose deaths.
Developing dosing guidelines for naloxone products is challenging; numerous prescription and illicit opioid compounds cause death by overdose, including multiples forms of fentanyl, which account for 150 deaths per day.3 These opioid compounds differ in how quickly they bind to and dissociate from opioid receptors in the brain, their potency, and how readily their effects can be reversed by a given dose of naloxone. Furthermore, the changes in respiration in an individual experiencing an opioid overdose reflect complex effects involving opioid receptor-medicated signaling, respiratory centers in the brain, breathing, and cardiac activity and circulation. Translational models constructed from available data that incorporate physiological information of how opioids and naloxone act in the body can be valuable tools to predict responses in a variety of overdose and intervention scenarios and for learning how opioid antagonists should be used.
Development and Validation of the Translational Model
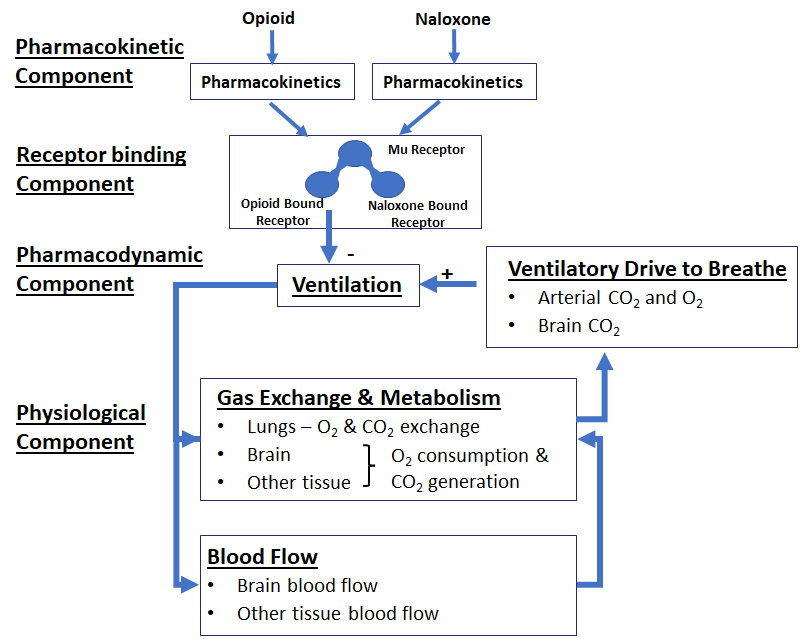
CDER scientists have developed, validated, and published such a translational model and are utilizing the framework for evaluating the effectiveness of different opioid antagonists and dosing strategies.4 This model has four components (Figure 1). First, a pharmacokinetic component based on available clinical data on plasma opioid levels relates doses of opioids and naloxone through different routes of administration to patient exposure to these drugs. Next, a receptor binding component relates changes in available opioid concentrations to occupancy of the receptors in the brain that mediate opioid effects on the central nervous system. Critical parameters of opioid binding to these receptors were derived from in vitro experiments conducted by CDER investigators and their collaborators at the Department of Veterans Affairs using fentanyl and multiple fentanyl derivatives as well as buprenorphine and naloxone. Finally, a wide variety of clinical and in vivo animal data were used to develop the pharmacodynamic and physiological components of the model to account for changes in air exchange by the lungs, arterial oxygen and carbon dioxide levels, and changes in total and cerebral blood circulation and cardiac activity. Only data that were withheld during model calibration were used to validate the CDER model, a step that greatly increases the confidence with which the model predictions could be used in regulatory decision making.
The CDER model effectively reproduced published clinical data, and data gathered during the model’s development provided additional insights into the mechanisms behind physiologic outcomes. For example, the drug buprenorphine, which is known to cause respiratory depression that is difficult to reverse, was found in the in vitro experiments to have a slow rate of dissociation from the opioid receptor. The model was also able to simulate patient respiratory responses in realistic scenarios (e.g., under levels of oxygen and carbon dioxide that would occur while breathing room air). The model also captured the patient’s cardiac response to a reduction in pulmonary ventilation, an important consideration when assessing potential outcomes from an opioid overdose and different intervention strategies. The inclusion of brain tissue oxygen levels allowed CDER scientists to assess the possibility of brain damage caused by overdose in situations where cardiac arrest does not occur.
Application of the Translational Model to Facilitate Drug Development
Recently, the Department of Defense (DOD), in collaboration with Kaléo, Inc., pursued development of a higher strength naloxone formulation suitable for use by military personnel and chemical incidence responders where use of high-potency opioids, such as fentanyl analogues, as a chemical weapon was suspected. Currently available opioid overdose reversal products were not specifically indicated for the DOD’s need. The DOD also noted that intranasal products cannot be used while wearing personal protective equipment and preferred auto-injectors for ease of administration by service members. Therefore, FDA received an NDA for a new 10 mg autoinjector naloxone formulation (Naloxone Auto-injector 10 mg; Kaléo) as part of medical countermeasures.
Demonstration of effectiveness for a new naloxone formulation for opioid overdose typically relies on a comparison of naloxone pharmacokinetics between the new formulation and an approved naloxone formulation, so that no new clinical efficacy studies are required. Given the indication for this new naloxone formulation and the lack of clinical data, additional quantitative investigations were requested of the sponsor to understand the capacity for this naloxone formulation to reverse effects of opioid exposure under different scenarios.
Although the sponsor had conducted simulations based on their quantitative model in support of the product, there remained important uncertainties about how effective the product would be for its intended use. In particular, when modeling the response of a patient to administration of the product, the sponsor model assumed a constant level of carbon dioxide in the lungs, but this would not be the case in a person breathing room air. Furthermore, because the endpoint of respiratory depression was based on the rate of air exchange in the lungs, the effects of opioid-induced changes in cardiac activity and circulation were not taken into account.
CDER scientists conducted independent modeling and simulation using the translational model to understand the capacity for this naloxone formulation to reverse effects of opioid exposure under different scenarios. This translational model and alternate endpoints of cardiac outcomes facilitated discussion within the review team and were critical in helping to understand the impact of the administered opioid, amount of the opioid, amount of naloxone, and timing of naloxone on recovery. Based on FDA’s translational model of patient response to opioids and naloxone, CDER was able to approve this NDA as an emergency treatment for individuals who are over aged 12 years where use of high-potency opioids, such as fentanyl analogues, as a chemical weapon is suspected. This model also provides CDER scientists with a robust quantitative framework for evaluating dosing regimens of opioid receptor antagonists to reverse opioid-induced respiratory depression in a community setting, including for newly emerging synthetic opioids with limited clinical data.
How does this work help the FDA address the opioid crisis?
It is challenging to assess naloxone dosing strategies in the community setting, including those necessary to reverse the respiratory depression effects of fentanyl and its derivatives. This translational model, developed by CDER scientists, presents a robust framework for evaluating the ability of dosing regimens of opioid receptor antagonists, not only to reverse opioid overdose in the community setting, but also to confront emerging synthetic opioids with limited clinical data.
References
- Centers for Disease Control and Prevention. (June 2, 2022). Death Rate Maps & Graphs. Retrieved September 30, 2022
- Caves, JP. Fentanyl as a Chemical Weapon. CSWMD Proceedings, December 2019. Retrieved November 8, 2022
- Centers for Disease Control and Prevention. (February 23, 2022). Fentanyl Facts. Retrieved November 8, 2022
- Mann J, Samieegohar M, Chaturbedi A, Zirkle J, Han X, Ahmadi SF, Eshleman A, Janowsky A, Wolfrum K, Swanson T, Bloom S, Dahan A, Olofsen E, Florian J, Strauss DG, Li Z. Development of a Translational Model to Assess the Impact of Opioid Overdose and Naloxone Dosing on Respiratory Depression and Cardiac Arrest. Clin Pharmacol Ther. 2022 Jun 29. doi: 10.1002/cpt.2696. Epub ahead of print.
Glossary
respiratory depression: a potentially fatal condition in which ventilation of the lungs is inadequate to perform needed exchange of oxygen and carbon dioxide.
naloxone: an opioid antagonist that binds opioid receptors and reverses and blocks the effects of other opioids.
autoinjector product: a medical device consisting of prefilled syringes or cartridges which are driven by a spring system that delivers drugs through subcutaneous or intramuscular routes.
fentanyl: a synthetic opioid that is similar to morphine but is 50 to 100 times more potent. Fentanyl is a prescription drug that is also made and used illegally.
opioid receptors: proteins in the outer membranes of nerve cells (neurons). When opioids bind to these receptors, the interaction triggers a series of neurochemical signals that lead to complex opioid effects including feelings of pleasure, pain relief, and respiratory depression.
translational model: a mathematical model consisting of a series of equations that describe temporal changes in drug concentrations in various compartments of the body and in some cases the time courses of various effects of these changes on physiologic processes.
computer simulation: the use of a computer to represent the dynamic responses of a system based on a model (for example a translational model of drug exposure and patient response). Computer outputs capture the behavior over time of the system (for example, in terms of changes in critical parameters [e.g., drug concentrations] and biochemical and physiological changes)