Drug Trials Snapshots: LITFULO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the LITFULO Prescribing Information for all the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
LITFULO (ritlecitinib)
lit-FUL-oh
Pfizer
Approval date: June 23, 2023
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
LITFULO is a kinase inhibitor that is indicated for the treatment of severe alopecia areata in adults and adolescents 12 years and older.
How is this drug used?
LITFULO is a capsule taken by mouth once a day.
Who participated in the clinical trials?
The FDA approved LITFULO based on evidence from a clinical trial of 718 patients with severe alopecia areata. The trial was conducted at 128 sites in 18 countries in Argentina, Australia, Canada, Chile, China, Colombia, Czech Republic, Germany, Hungary, Japan, Republic of Korea, Mexico, Poland, Russian Federation, Spain, Taiwan, the United Kingdom, and the United States. The safety evaluation was also supported by two clinical trials that enrolled a total of 162 patients with alopecia areata who were treated with LITFULO or placebo.
How were the trials designed?
The efficacy and safety of LITFULO were evaluated in a randomized, double-blind, placebo controlled trial in 718 patients 12 years of age and older with alopecia areata with ≥50% scalp hair loss, including alopecia totalis and alopecia universalis. The trial randomized 130 patients to LITFULO 50 mg once daily, 131 patients to placebo, and 457 patients to other ritlecitinib dosing regimens.
The safety evaluation was also supported by two placebo-controlled trials in which 80 patients were randomized to LITFULO 200 mg once daily for four weeks followed by 50 mg once daily and 82 patients were randomized to placebo.
Assessment of scalp hair loss was based on the Severity of Alopecia Tool (SALT) score. SALT scores range from 0 to 100 with 0 = no scalp hair loss and 100 = total scalp hair loss. The primary endpoint was the proportion of patients with SALT ≤20 response (20% or less of scalp hair loss) at Week 24.
How were the trials designed?
The efficacy and safety of LITFULO were evaluated in a randomized, double-blind, placebo controlled trial in 718 patients 12 years of age and older with alopecia areata with ≥50% scalp hair loss, including alopecia totalis and alopecia universalis.
The trial randomized patients to one of the following treatment regimens for 48 weeks: 1) LITFULO 200 mg once daily for 4 weeks followed by 50 mg once daily for 44 weeks (132 patients); 2) LITFULO 200 mg once daily for 4 weeks followed by 30 mg once daily for 44 weeks (130 patients); 3) LITFULO 50 mg once daily for 48 weeks (130 patients); 4) LITFULO 30 mg once daily for 48 weeks (132 patients); 5) LITFULO 10 mg once daily for 48 weeks (63 patients); 6) placebo for 24 weeks followed by LITFULO 200 mg once daily for 4 weeks and 50 mg once daily for 20 weeks (65 patients); or 7) placebo for 24 weeks followed by LITFULO 50 mg once daily for 24 weeks (66 patients). Safety was also evaluated in two placebo-controlled trials in which patients were randomized to LITFULO 200 mg once daily for four weeks followed by 50 mg once daily (80 patients) or placebo (82 patients).
The safety evaluation was also supported by two randomized placebo-controlled trials in which 80 patients were randomized to LITFULO 200 mg once daily for 4 weeks followed by 50 mg once daily and 82 patients were randomized to placebo for 24 weeks.
Assessment of scalp hair loss was based on SALT score. SALT scores range from 0 to 100 with 0 = no scalp hair loss and 100 = total scalp hair loss. The primary endpoint was the proportion of patients with SALT ≤20 response (20% or less of scalp hair loss) at Week 24 and the secondary endpoint was the proportion of patients with SALT ≤10 response (10% or less of scalp hair loss) at Week 24.
DEMOGRAPHICS SNAPSHOT:
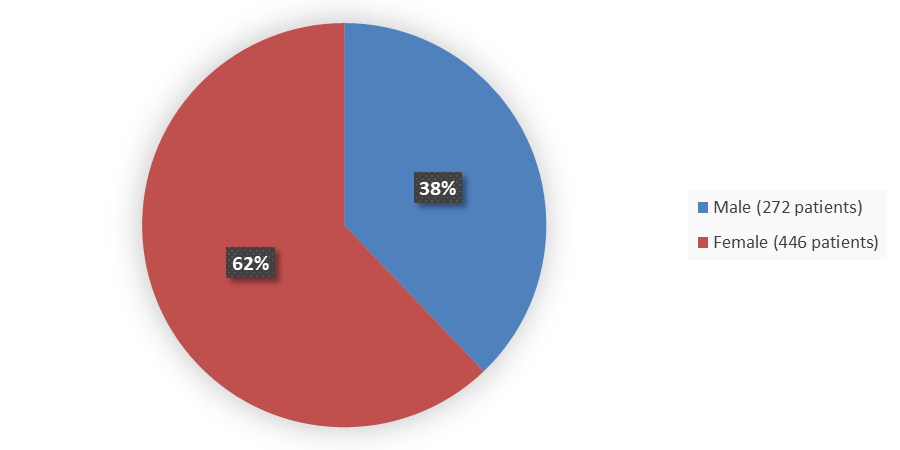
Figure 1 summarizes how many male and female patients were enrolled in the clinical trial used to evaluate the efficacy of LITFULO.
Figure 1. Baseline Demographics by Sex
Source: Adapted from FDA Review
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trial used to evaluate the efficacy of LITFULO.
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA Review
*Includes American Indian or Alaskan Native, Native Hawaiian or Other Pacific Islander, multiracial, and race not reported
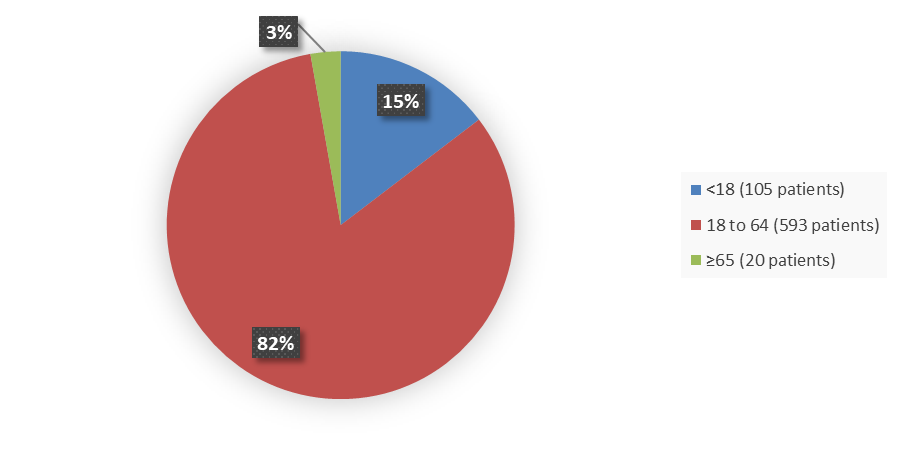
Figure 3 summarizes the percentage of patients by age enrolled in the clinical trial used to evaluate the efficacy of LITFULO.
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Review
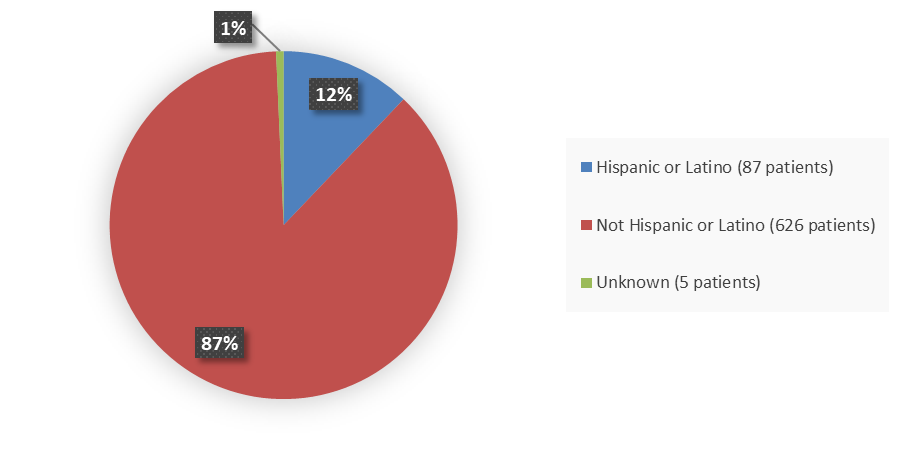
Figure 4 summarizes the percentage of patients by ethnicity enrolled in the clinical trial used to evaluate the efficacy of LITFULO.
Figure 4. Baseline Demographics by Ethnicity
Source: Adapted from FDA Review
Who participated in the trials?
Table 1 summarizes the demographics in the trial.
Table 1. Trial Demographics
| Demographic |
LITFULO N=130 |
Other Ritlecitinib Regimens N=457 |
Placebo N=131 |
|
Age, years |
|
|
|
|
<18 |
18 (14) |
68 (15) |
19 (15) |
|
18 to 64 |
109 (84) |
377 (82) |
107 (82) |
|
≥65 |
3 (2) |
12 (3) |
5 (4) |
|
Gender |
|
|
|
|
Female |
71 (55) |
289 (63) |
86 (66) |
|
Male |
59 (45) |
168 (37) |
45 (34) |
|
Race |
|
|
|
|
White |
79 (61) |
315 (69) |
94 (72) |
|
Black or African American |
5 (4) |
18 (4) |
4 (3) |
|
Asian |
43 (33) |
112 (25) |
31 (24) |
|
Other* |
3 (2) |
12 (3) |
2 (2) |
|
Ethnicity |
|
|
|
|
Hispanic or Latino |
11 (8) |
65 (14) |
11 (8) |
|
Not Hispanic or Latino |
116 (89) |
391 (86) |
119 (91) |
|
Unknown |
3 (2) |
1 (<1) |
1 (1) |
Source: Adapted from FDA Review
*Includes American Indian or Alaskan Native, Native Hawaiian or Other Pacific Islander, multiracial, and race not reported
What are the benefits of this drug?
More patients achieved 20% or less scalp hair loss on LITFULO compared to placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2 summarizes the efficacy results for the patients evaluated in a clinical trial for LITFULO. Assessment of scalp hair loss was based on SALT score. SALT scores range from 0 to 100 with 0 = no scalp hair loss and 100 = total scalp hair loss. At Week 24, a greater proportion of subjects had a SALT ≤20 response (20% or less of scalp hair loss) and SALT ≤10 response (10% or less of scalp hair loss) with LITFULO compared to placebo.
Table 2. Summary of Sitting FVC in Adults With LOPD by ERT Status at 52 Weeks in Trial 1
|
Parameter |
LITFULO N=130 % Responders |
Placebo N=131 % Responders |
Difference From Placebo (95% CI) |
|
SALT ≤20 response |
23.0 |
1.6 |
21.4 (13.4, 29.5) |
|
SALT ≤10 response |
13.4 |
1.5 |
11.9 (5.4, 18.3) |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval; SALT, Severity of Alopecia Tool
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: LITFULO worked better in females than males.
- Race: LITFULO worked similarly in White and Asian patients. The number of patients of races other than White and Asian was limited; therefore, differences in response among races could not be determined.
- Age: LITFULO worked similarly in those <18 years of age and those 18 to 64 years of age. The number of patients older than 65 years of age was limited; therefore, differences in response between patients younger and older than 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
Table 3. SALT ≤20 at Week 24 by Subgroup
|
Subgroup |
LITFULO N=130 n/N (%) |
Placebo N=131 n/N (%) |
Difference From Placebo 95% CI |
|
Age, years |
|
|
|
|
<18 |
4/16 (25) |
0/19 (0) |
6, 50 |
|
18 to 64 |
24/105 (23) |
1/106 (1) |
14, 31 |
|
≥65 |
1/3 (33) |
1/5 (20) |
-46, 70 |
|
Sex |
|
|
|
|
Female |
25/69 (36) |
2/85 (2) |
24, 46 |
|
Male |
4/55 (7) |
0/45 (0) |
-1, 17 |
|
Race |
|
|
|
|
White |
18/74 (24) |
1/93 (1) |
14, 34 |
|
Black or African American |
0/5 (0) |
0/4 (0) |
NA |
|
Asian |
10/42 (24) |
1/31 (3) |
5, 36 |
|
Multiracial or not reported |
1/3 (33) |
0/2 (0) |
NA |
|
Ethnicity |
|
|
|
|
Hispanic or Latino |
5/11 (45) |
0/11 (0) |
13, 73 |
|
Not Hispanic or Latino |
24/110 (22) |
2/118 (2) |
13, 29 |
|
Unknown |
0/3 (0) |
0/1 (0) |
NA |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval; NA, not applicable
What are the possible side effects?
Most common side effects include headache, diarrhea, acne, rashes, eczema, fever, mouth ulcers, dizziness, shingles rash, and abnormal findings in some laboratory test results.
Serious infections, malignancies, blood clots, allergic reactions, and abnormal laboratory findings (low white blood cells [lymphocytes], low platelets, high muscle enzymes [CPK], and high liver enzyme) has been reported in some patients taking LITFULO.
What are the possible side effects (results of trials used to assess safety)?
Adverse events reported in ≥1% of patients treated with LITFULO and more common than patients treated with placebo up to Week 24 are listed Table 4.
Table 4. Adverse Reactions in Clinical Trials of LITFULO for the Treatment of Alopecia Areataa
| Adverse Reaction |
LITFULO N=130 n (%) |
Placebo N=213 n (%) |
|
Headacheb |
14 (10.8) |
18 (8.5) |
|
Diarrheac |
13 (10.0) |
8 (3.8) |
|
Acned |
8 (6.2) |
10 (4.7) |
|
Rashe |
7 (5.4) |
2 (0.9) |
|
Urticaria |
6 (4.6) |
3 (1.4) |
|
Folliculitis |
4 (3.1) |
4 (1.9) |
|
Pyrexia |
4 (3.1) |
0 |
|
Dermatitis atopic |
3 (2.3) |
1 (0.5) |
|
Dizziness |
3 (2.3) |
3 (1.4) |
|
Blood creatine phosphokinase increased |
2 (1.5) |
0 |
|
Herpes zoster |
2 (1.5) |
0 |
|
Red blood cell count decreased |
2 (1.5) |
0 |
|
Stomatitis |
2 (1.5) |
0 |
Source: LITFULO Prescribing Information
a Reported in ≥1% of subjects and at a higher rate than placebo for up to 24 weeks.
b Headache includes headache and migraine.
c Diarrhea includes diarrhea and frequent bowel movements.
d Acne includes acne and acne pustular.
e Rash includes rash and dermatitis allergic.
Were there any differences in side effects among sex, race, and age?
- Sex: The occurrence of side effects was more common in females than males.
- Race: The occurrence of side effects was similar in White and Asian patients. The number of patients of races other than White and Asian was limited; therefore, differences in response among races could not be determined.
- Age: The number of patients older than 65 years of age was limited; therefore, differences in adverse event rates between patients younger and older than 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
The overall safety database and individual subgroup sample sizes were not sufficient to detect clinically meaningful differences in the frequency of treatment-emergent adverse events (TEAEs) between individual subgroups.
Age subgroup: No clinically meaningful differences between the adverse event (AE) profiles for adolescent and adult subjects were identified. A trend towards a higher frequency of reported TEAEs (urinary tract infection, lymphocyte count decreased, arthralgia, and herpes zoster), severe AEs, serious adverse events (SAEs) (including serious infections), AE leading to discontinuations (AELD)s, and adjudicated AEs of malignancy (excluding nonmelanoma skin cancer and basal cell carcinoma) in subjects ≥65 years of age, compared to adult subjects (≥18 years of age) were reported for the all-exposure pool.
Sex subgroup: Female subjects were reported with a higher frequency of TEAEs and with similar frequency for severe AEs, SAEs, AELDs, and adverse reactions (related) (ARs) than male subjects in their corresponding LITFULO dose groups (and placebo group). No clinically meaningful differences between the AE profiles for male and female subjects were identified (including safety AEs of interest). Headache and urinary tract infections were higher, and blood creatine phosphokinase increased lower in female patients.
Race and ethnicity subgroups: Subgroup analysis by race included only White and Asian subjects due to limited number of subjects in other race groups. Asian subjects were reported with a higher frequency of upper respiratory tract infections, folliculitis, and urticaria in the LITFULO 50 mg dose group. Hispanic or Latino subjects compared to not Hispanic or Latino subjects showed more frequent TEAEs in the all-exposure pool.
Table 5. Proportion of Subjects With Treatment-Emergent Adverse Events by Subgroup Through Week 24
|
Subgroup |
LITFULO n/Ns (%) |
Placebo n/Ns (%) |
|
Age, years |
|
|
|
<18 |
15/18 (83) |
15/19 (79) |
|
≥18 |
83/112 (74) |
78/112(69) |
|
Sex |
|
|
|
Female |
59/71 (83) |
67/86 (78) |
|
Male |
39/59 (66) |
26/45 (58) |
|
Race |
|
|
|
White |
59/79 (75) |
67/94 (71) |
|
Asian |
33/43 (77) |
21/31(68) |
Source: Adapted from FDA Review
Abbreviations: n, number of patients meeting criteria; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
LINK TO DRUG PACKAGE INSERT: