Drug Trials Snapshots: ZURZUVAE
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the ZURZUVAE Prescribing Information for all the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
ZURZUVAE (zuranolone)
(ZUR-zoo-vay)
Biogen, Inc.
Approval date: August 4, 2023
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ZURZUVAE is a neuroactive steroid drug used for the treatment of postpartum depression in adults.
How is this drug used?
ZURZUVAE is given as capsules taken by mouth once daily in the evening with fat-containing food for 14 days.
Who participated in the clinical trials?
The FDA approved ZURZUVAE based on evidence from two clinical trials of 345 patients with postpartum depression. The trials were conducted at 125 sites in 3 countries in the United States, Spain, and the United Kingdom.
In Trials 1 and 2, the baseline demographics of patients were similar between the ZURZUVAE and placebo groups. In Trial 1, 98% of patients were enrolled in the United States; patients had a mean age of 30 years (range 19 to 44 years); 70% of patients were White, 22% Black or African American, 1% Asian, and 7% other races; and 38% of patients were of Hispanic or Latino ethnicity. In Trial 2, all patients were enrolled in the United States; patients had a mean age of 28 years (range 18 to 44 years); 56% of patients were White, 41% Black or African American, 1% Asian, and 2% other races; and 23% of patients were of Hispanic or Latino ethnicity.
The same trials were used to assess efficacy and safety. The number of patients representing efficacy findings may differ from the number of patients representing safety findings due to different pools of study participants analyzed for efficacy and safety.
How were the trials designed?
ZURZUVAE was evaluated in two clinical trials of 345 patients with postpartum depression.
Both trials enrolled patients 18 to 45 years old with postpartum depression who had an episode of major depression starting in the third trimester of pregnancy or within four weeks after delivering a baby. The investigator used the Hamilton Depression Rating Scale (HAM-D) to determine the patient’s level of depression. The HAM-D scores eight items on a 5-point grading scale (not present to severe). Both trials enrolled patients with severe postpartum depression (HAM-D score of ≥26), and use of another oral antidepressant at a stable dose was allowed. Patients in Trial 1 were randomized to receive ZURZUVAE 50 mg or placebo once daily for 14 days. Patients in Trial 2 were randomized to receive another zuranolone capsule formulation (approximately equal to ZURZUVAE 40 mg) or placebo once daily for 14 days. In both trials, neither the patients nor the health care providers knew which treatment was being given until after the trial was completed. The benefit of ZURZUVAE was assessed in both trials by determining the improvement in depressive symptoms (the difference in HAM-D scores before and after treatment).
How were the trials designed?
The benefit of ZURZUVAE was evaluated in two randomized, double-blind, placebo-controlled clinical trials in adult female patients (18 to 45 years) with postpartum depression who met Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria for a major depressive episode with onset of symptoms in the third trimester or within four weeks of delivery. Both trials included patients with severe postpartum depression (HAM-D score ≥26). Patients in Trial 1 were randomized to receive ZURZUVAE 50 mg, with the option to reduce the dosage based on tolerability to 40 mg once daily, or placebo once daily for 14 days. Patients in Trial 2 were randomized to receive another zuranolone capsule formulation (approximately equal to ZURZUVAE 40 mg) or placebo once daily for 14 days. The primary efficacy endpoint was the mean change from baseline in the HAM-D total score at the end of the treatment course (Day 15).
DEMOGRAPHICS SNAPSHOT
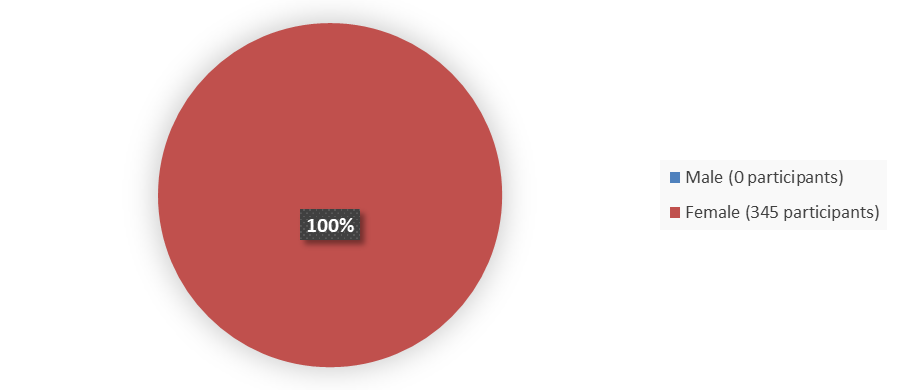
Figure 1 summarizes how many male and female patients were enrolled in the clinical trials used to evaluate the efficacy of ZURZUVAE.
Figure 1. Baseline Demographics by Sex
Source: Adapted from FDA Review
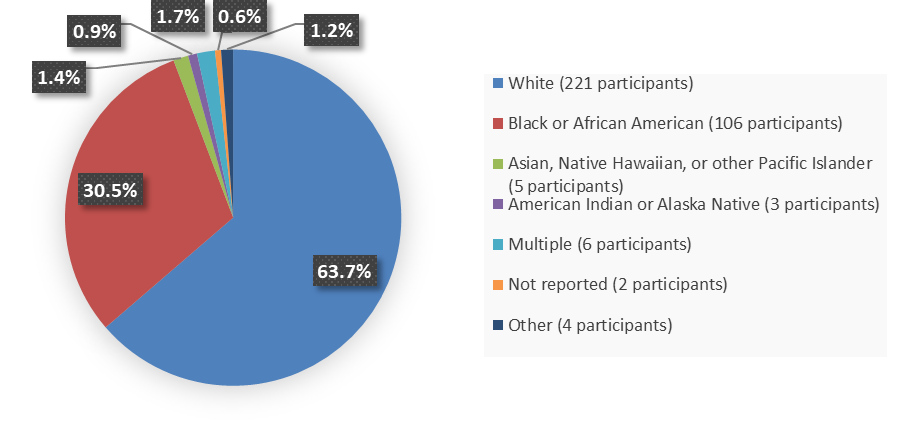
Figure 2 summarizes the percentage of patients by race were enrolled in the clinical trials used to evaluate the efficacy of ZURZUVAE.
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA Review
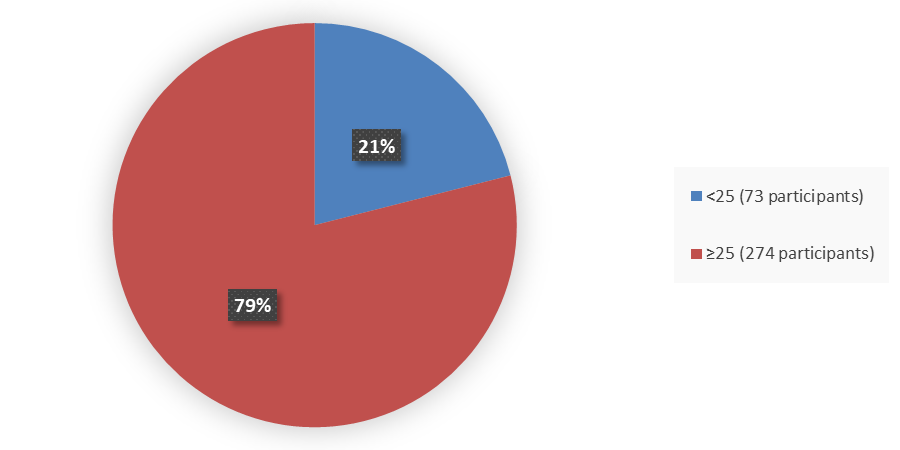
Figure 3 summarizes the percentage of patients by age were enrolled in the clinical trials used to evaluate the efficacy of ZURZUVAE.
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Review
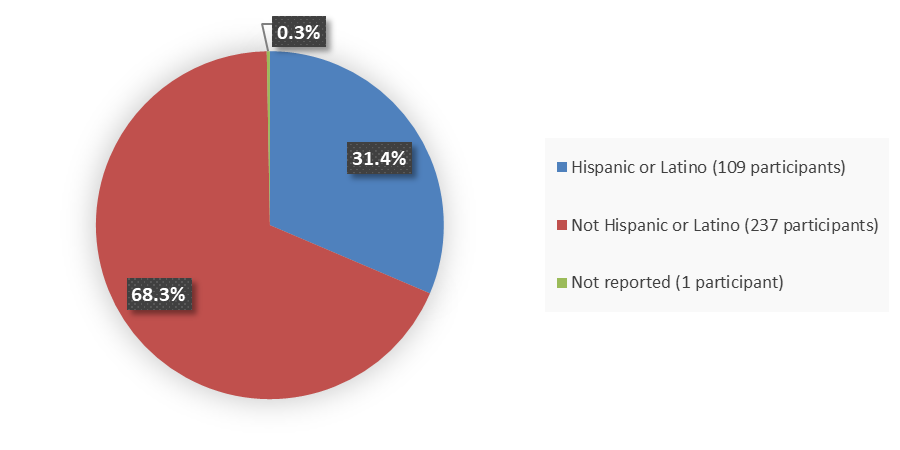
Figure 4 summarizes the percentage of patients by ethnicity were enrolled in the clinical trials used to evaluate the efficacy of ZURZUVAE.
Figure 4. Baseline Demographics by Ethnicity
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Trial 1 Baseline Demographics
| Characteristic | Placebo N=98 n (%) |
ZURZUVAE N=98 n (%) |
Total N=196 n (%) |
|---|---|---|---|
| Race | |||
| American Indian or Alaska Native | 3 (3.1) | 0 | 3 (1.5) |
| Asian | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| Black or African American | 18 (18.4) | 25 (25.5) | 43 (21.9) |
| Multiple | 2 (2.0) | 3 (3.1) | 5 (2.6) |
| Not reported | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| White | 69 (70.4) | 68 (69.4) | 137 (69.9) |
| Other | 4 (4.1) | 0 | 4 (2.0) |
| Age group, years | |||
| 18 to 24 | 12 (12.2) | 19 (19.4) | 31 (15.8) |
| 25 to 45 | 86 (87.8) | 79 (80.6) | 165 (84.2) |
| Ethnicity | |||
| Hispanic or Latino | 42 (42.9) | 33 (33.7) | 75 (38.3) |
| Not Hispanic or Latino | 56 (57.1) | 64 (65.3) | 120 (61.2) |
| Not reported | 0 | 1 (1.0) | 1 (<1) |
Source: Adapted from FDA Review
Table 2. Trial 2 Baseline Demographics
| Characteristic | Placebo N=73 n (%) |
Zuranolone* N=78 n (%) |
Total N=151 n (%) |
|---|---|---|---|
| Race | |||
| Asian | 1 (<1.0) | 1 (<1.0) | 2 (1.1) |
| Black or African American | 32 (43.8) | 31 (39.7) | 63 (41.7) |
| Multiple | 0 | 1 (<1.0) | 1 (<1.0) |
| Native Hawaiian or other Pacific Islander | 1 (<1.0) | 0 | 1 (<1.0) |
| White | 39 (53.4) | 45 (57.7) | 84 (55.6) |
| Age group, years | |||
| 18 to 24 | 25 (34.2) | 17 (21.8) | 42 (27.8) |
| 25 to 45 | 48 (65.8) | 61 (78.2) | 109 (72.2) |
| Ethnicity | |||
| Hispanic or Latino | 18 (24.7) | 16 (20.5) | 34 (22.5) |
| Not Hispanic or Latino | 57 (78.1) | 60 (76.9) | 117 (77.5) |
Source: Adapted from FDA Review
* This capsule formulation of zuranolone is approximately equivalent to ZURZUVAE 40 mg.
What are the benefits of this drug?
In two trials, patients with severe postpartum depression who were given ZURZUVAE achieved more improvement of depressive symptoms than patients given placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 3 summarizes efficacy results for the evaluated patients in the clinical trials. The primary efficacy endpoint was the change from baseline in the HAM-D total score at the end of the treatment course (Day 15).
Table 3. Results for the Primary Endpoint: Change From Baseline in the HAMD-17 Total Score at Day 15
| Trial | Treatment Group | N | Mean Baseline Score (SD) | LS Mean Change From Baseline (SE) | Treatment Difference (95% CI) |
|---|---|---|---|---|---|
| 1 | ZURZUVAE 50 mg | 98 | 28.6 (2.49) | -15.6 (0.82) | -4.0 (-6.3, -1.7) |
| Placebo | 97 | 28.8 (2.34) | -11.6 (0.82) | ||
| 2 | Zuranolone* | 76 | 28.4 (2.09) | -17.8 (1.04) | -4.2 (-6.9, -1.5) |
| Placebo | 74 | 28.8 (2.32) | -13.6 (1.07) |
Source: ZURZUVAE Prescribing Information
* This capsule formulation of zuranolone is approximately equivalent to ZURZUVAE 40 mg.
Abbreviations: CI, confidence interval; HAMD-17, 17-item Hamilton Depression Rating Scale; LS, least squares; SD, standard deviation; SE, standard error
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: All patients in the trials were female.
- Race: ZURZUVAE worked similarly in White and Black or African American patients. The number of patients in other races was limited; therefore, differences in how well ZURZUVAE worked in other races could not be determined.
- Age: Fewer patients younger than 25 years old were enrolled in the studies. Differences in how well ZURZUVAE worked in patients younger and older than 25 years of age could not be determined. All patients were younger than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 4 and Table 5 summarize efficacy results by race and age based on the mean change from baseline in the HAM-D total score at the end of the treatment course (Day 15). Of note, the comparisons of race and age were not powered to determine differences between groups for changes in the HAM-D total score.
Table 4. Trial 1 Efficacy Results by Race and Age
| Variable | N | Placebo LS Mean (SE) Change From Baseline | ZURZUVAE LS Mean (SE) Change From Baseline | Treatment Difference (95% CI) | P-value |
|---|---|---|---|---|---|
| Overall | 183 | -11.6 (0.82) | -15.6 (0.82) | -4 (-6.3, -1.7) | 0.0007 |
| Race | |||||
| Black or African American | 36 | -11.5 (2.18) | -17.5 (1.77) | -6 (-11.8, -0.2) | 0.0424 |
| White | 132 | -11.9 (0.94) | -15.4 (0.94) | -3.5 (-6.2, -0.9) | 0.0089 |
| Age, years | |||||
| 18 to 24 | 31 | -13.9 (2.25) | -18.8 (1.75) | -4.9 (-10.9, 1) | 0.1023 |
| 25 to 45 | 152 | -11.2 (0.89) | -14.9 (0.92) | -3.6 (-6.2, -1.1) | 0.005 |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval; LS, least squares; SE, standard error
Table 5. Trial 2 Efficacy Results by Race and Age
| Variable | N | Placebo LS Mean (SE) Change From Baseline | Zuranolone* LS Mean (SE) Change From Baseline | Treatment Difference (95% CI) | P-value |
|---|---|---|---|---|---|
| Race | |||||
| Black or African American | 62 | -13.2 (1.59) | -18 (1.59) | -4.7 (-9.3, -0.2) | 0.0404 |
| White | 81 | -13.7 (1.3) | -17.4 (1.25) | -3.7 (-7.3, -0.1) | 0.0448 |
| Age, years | |||||
| 18 to 24 | 42 | -14 (1.68) | -16.5 (2.04) | -2.5 (-7.9, 2.8) | 0.3458 |
| 25 to 45 | 105 | -13.3 (1.2) | -18.1 (1.1) | -4.8 (-8, -1.5) | 0.0041 |
Source: Adapted from FDA Review
* This capsule formulation of zuranolone is approximately equivalent to ZURZUVAE 40 mg.
Abbreviations: CI, confidence interval; LS, least squares; SE, standard error
What are the possible side effects?
ZURZUVAE may cause serious side effects such as decreased ability to drive or do other dangerous activities, decreased awareness and alertness (sleepiness and confusion), increased risk of suicidal thoughts or actions, and risk of harm to the fetus while pregnant. The most common side effects in the trials were sleepiness or drowsiness; dizziness; diarrhea; feeling tired, weak, or having no energy; common cold; and urinary tract infection.
What are the possible side effects (results of trials used to assess safety)?
Table 6 and Table 7 summarize adverse reactions in patients with severe postpartum depression in the clinical trials.
Table 6. Trial 1 Adverse Reactions That Occurred in ≥2% of Patients With Postpartum Depression Treated With ZURZUVAE 50 mg and Greater Than in Patients Treated With Placebo
| Adverse Reaction | Placebo N=98 % |
ZURZUVAE N=98 % |
|---|---|---|
| Somnolence1 | 6 | 36 |
| Dizziness2 | 9 | 13 |
| Diarrhea | 2 | 6 |
| Fatigue3 | 2 | 5 |
| Urinary tract infection | 4 | 5 |
| Memory impairment | 0 | 3 |
| Abdominal pain4 | 0 | 3 |
| Tremor | 0 | 2 |
| Hypoesthesia | 0 | 2 |
| Muscle twitching | 0 | 2 |
| Myalgia | 0 | 2 |
| COVID-19 | 0 | 2 |
| Anxiety | 1 | 2 |
| Rash | 1 | 2 |
Source: ZURZUVAE Prescribing Information
1Somnolence includes sedation and hypersomnia
2Dizziness includes vertigo
3Fatigue includes asthenia
Table 7. Trial 2 Adverse Reactions That Occurred in ≥2% of Patients With Postpartum Depression Treated With Zuranolone* and Greater Than in Patients Treated With Placebo
| Adverse Reaction | Placebo N=73 % |
Zuranolone* N=78 % |
|---|---|---|
| Somnolence1 | 11 | 19 |
| Nasopharyngitis2 | 3 | 9 |
| Dizziness | 6 | 8 |
| Fatigue3 | 1 | 5 |
| Diarrhea | 3 | 5 |
| Dry mouth | 0 | 4 |
| Sinus congestion | 0 | 3 |
| Toothache | 0 | 3 |
Source: ZURZUVAE Prescribing Information
* This capsule formulation of zuranolone is approximately equivalent to ZURZUVAE 40 mg.
1 Somnolence includes sedation
2 Nasopharyngitis includes upper respiratory tract infection
3 Fatigue includes lethargy
Were there any differences in side effects among sex, race and age?
- Sex: All patients in the trials were female.
- Race: In Trial 1, the occurrence of side effects was better in Black or African American patients. In Trial 2, the occurrence of side effects was better in White patients. Given these differences in Trials 1 and 2 and the limited number of patients in other races, differences in the occurrence of side effects among races could not be determined.
- Age: The occurrence of side effects was better in patients aged 25 years and older. However, the number of patients in the younger than 25-year-old age subgroup was limited; therefore, differences in the occurrence of side effects among age subgroups should be interpreted with caution.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 8 and Table 9 summarize the occurrence of adverse reactions by sex, age group, race, and ethnicity in the safety population.
Table 8. Trial 1 Overview of Adverse Events by Demographic Subgroup
| Characteristic | ZURZUVAE N=98 n/Ns (%) |
Placebo N=98 n/Ns (%) |
Risk Difference % (95% CI) |
|---|---|---|---|
| Sex | |||
| Female | 60/98 (61.2) | 44/98 (44.9) | 16.3 (2.5, 30.1)1 |
| Age group, years | |||
| 18 to 24 | 16/19 (84.2) | 6/12 (50.0) | 34.2 (1.5, 66.9)1 |
| 25 to 45 | 44/79 (55.7) | 38/86 (44.2) | 11.5 (‑3.7, 26.7) |
| Race | |||
| American Indian or Alaska Native | 0/0 (N/A) | 2/3 (66.7) | N/A |
| Asian | 0/1 (0) | 1/1 (100) | N/A |
| Black or African American | 14/25 (56.0) | 9/18 (50.0) | 6.0 (‑24.2, 36.2) |
| Multiple | 1/3 (33.3) | 0/2 (0) | 33.3 (‑20.0, 86.7) |
| Native Hawaiian or other Pacific Islander | 0/0 (N/A) | 0/0 (N/A) | N/A |
| Not reported | 1/1 (100) | 1/1 (100) | 0 (0, 0) |
| Other | 0/0 (N/A) | 3/4 (75.0) | N/A |
| White | 44/68 (64.7) | 28/69 (40.6) | 24.1 (7.9, 40.4)a |
| Ethnicity | |||
| Hispanic or Latino | 21/33 (63.6) | 20/42 (47.6) | 16.0 (‑6.3, 38.3) |
| Not Hispanic or Latino | 38/64 (59.4) | 24/56 (42.9) | 16.5 (‑1.2, 34.2) |
| Not reported | 1/1 (100) | 0/0 (N/A) | N/A |
| Is in United States | |||
| United States | 60/98 (61.2) | 44/98 (44.9) | 16.3 (2.5, 30.1)1 |
Source: Adapted from FDA Review
a Indicates rows where the 95% confidence interval excludes zero.
Abbreviations: CI, confidence interval; N, number of subjects in treatment arm; n, number of subjects with adverse event; N/A, not applicable; Ns, total number of subjects for each specific subgroup and were assigned to that specific arm
Table 9. Trial 2 Overview of Adverse Events by Demographic Subgroup
| Characteristic | Zuranolone* N=78 n/Ns (%) |
Placebo N=73 n/Ns (%) |
Risk Difference % (95% CI) |
|---|---|---|---|
| Sex | |||
| Female | 44/78 (56.4) | 34/73 (46.6) | 9.8 (‑6.0, 25.7) |
| Age group, years | |||
| 18 to 24 | 9/17 (52.9) | 10/25 (40.0) | 12.9 (‑17.6, 43.5) |
| 25 to 45 | 35/61 (57.4) | 24/48 (50.0) | 7.4 (‑11.4, 26.2) |
| Race | |||
| American Indian or Alaska Native | 0/0 (N/A) | 0/0 (N/A) | N/A |
| Asian | 1/1 (100) | 1/1 (100) | 0 (0, 0) |
| Black or African American | 22/31 (71.0) | 9/32 (28.1) | 42.8 (20.5, 65.2)a |
| Multiple | 1/1 (100) | 0/0 (N/A) | N/A |
| Native Hawaiian or other Pacific Islander | 0/0 (N/A) | 1/1 (100) | N/A |
| Not reported | 0/0 (N/A) | 0/0 (N/A) | N/A |
| White | 20/45 (44.4) | 23/39 (59.0) | -14.5 (‑35.7, 6.7) |
| Ethnicity | |||
| Hispanic or Latino | 7/18 (38.9) | 8/16 (50.0) | -11.1 (‑44.4, 22.2) |
| Not Hispanic or Latino | 37/60 (61.7) | 26/57 (45.6) | 16.1 (‑1.8, 33.9) |
| Not reported | 0/0 (N/A) | 0/0 (N/A) | N/A |
| Is in United States | |||
| United States | 44/78 (56.4) | 34/73 (46.6) | 9.8 (‑6.0, 25.7) |
Source: Adapted from FDA Review
* This capsule formulation of zuranolone is approximately equivalent to ZURZUVAE 40 mg.
a Indicates rows where the 95% confidence interval excludes zero.
Abbreviations: CI, confidence interval; N, number of subjects in treatment arm; n, number of subjects with adverse event; N/A, not applicable; Ns, total number of subjects for each specific subgroup and were assigned to that specific arm
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION