Laboratory Accreditation for Analyses of Foods (LAAF) Program & Final Rule
Program | Application, Portal, & Dashboard
Final Rule | Resources | FAQs | Contact Us
Voluntary Graphic Element & QR Code
Laboratory Accreditation for Analyses of Foods Program
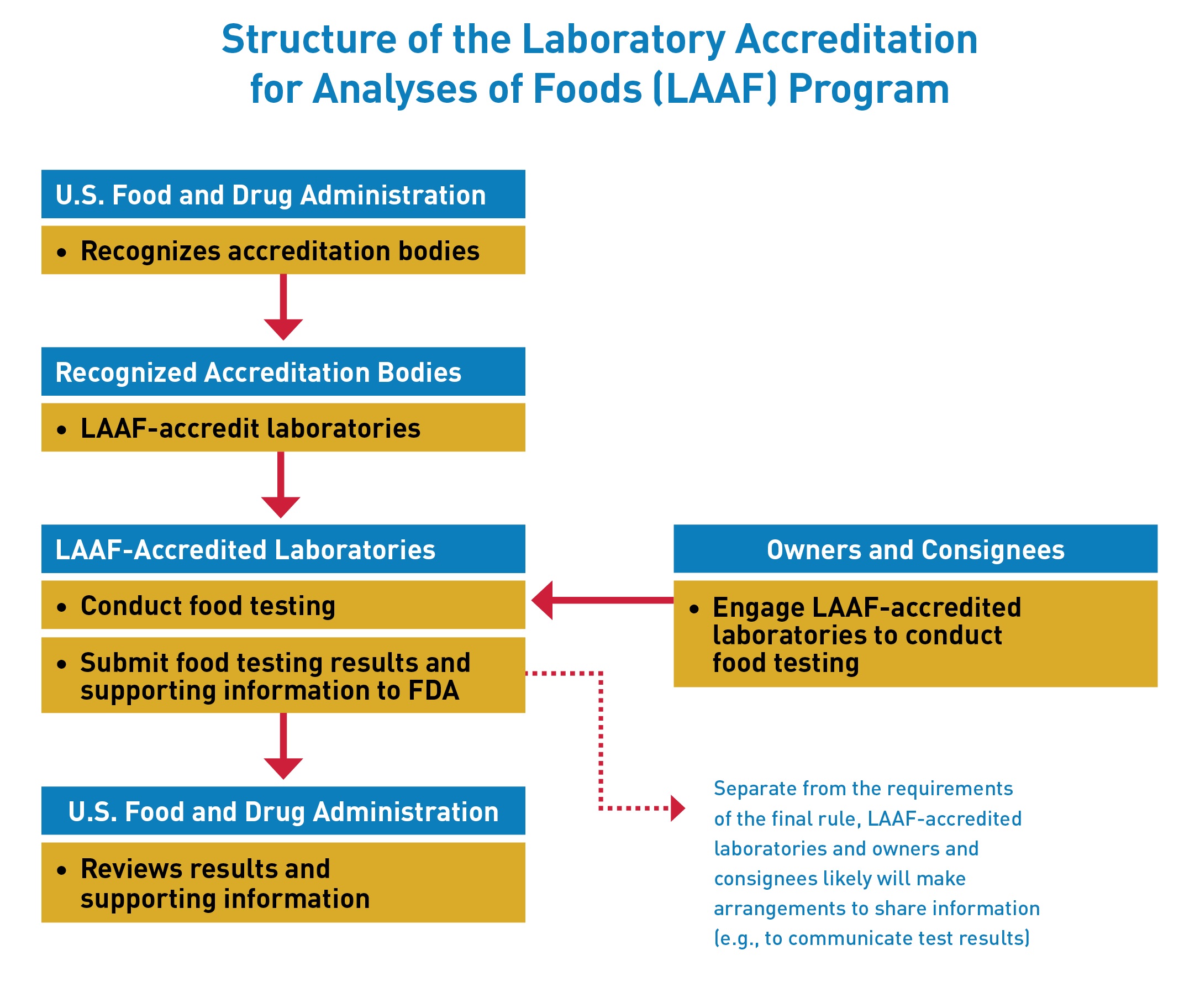
The Laboratory Accreditation for Analyses of Foods (LAAF) Program establishes a laboratory accreditation program for the testing of food in certain circumstances. Under the LAAF program, FDA will recognize “accreditation bodies” that will have the responsibility of accrediting “LAAF-accredited laboratories” to the standards established by the rule. In certain circumstances, owners and consignees will be required to use a LAAF-accredited laboratory for food testing.
The establishment of the LAAF program is intended to improve the accuracy and reliability of certain food testing through the uniformity of standards and enhanced FDA oversight of participating laboratories.
Enlarge in PDF (241KB)
Recognized Accreditation Bodies may accredit laboratories to conduct food testing under this program once they receive recognition from FDA.
Testing laboratories may apply directly to one of the recognized accreditation bodies to seek LAAF-accreditation.
Owners and consignees will be required to use a LAAF-accredited laboratory for food testing:
- to support removal of a food from an import alert through successful consecutive testing requirements;
- to support admission of an imported food detained at the border because it is or appears to be in violation of the Federal Food, Drug, and Cosmetic Act;
- required by existing FDA food safety regulations, when applied to address an identified or suspected food safety problem (i.e., certain tests of shell eggs, sprouts, and bottled drinking water);
- required by a directed food laboratory order, a new procedure being implemented in this final rule that will allow FDA to require use of a LAAF-accredited laboratory to address an identified or suspected food safety problem in certain, rare circumstances; and
- conducted in connection with certain administrative processes such as testing
The FDA has determined that there is sufficient laboratory capacity in the LAAF program for the import-related food testing covered by the LAAF regulation (§ 1.1107(a)(4) and (5)) for those analyte groups listed on the LAAF Dashboard LAAF-Compliance Dates Table located at: FDA Dashboards - Laboratory Accreditation for Analyses of Foods Program. As sufficient capacity is reached for additional analytes covered under the import-related food testing provisions of the LAAF regulation, those specific analytes and compliance dates will be posted on the LAAF Dashboard. Owners and consignees of imported food subject to the LAAF regulation must use a LAAF-accredited laboratory to conduct covered import-related food testing starting on the applicable compliance date, which is 6 months from the date a specific analyte is listed on a public registry, based on FDA’s determination that sufficient laboratory capacity has been achieved for such analyte.
We will continue stepwise implementation of the LAAF program for other food testing circumstances in which owners and consignees are required to use a LAAF-accredited laboratory. FDA has not yet made a capacity determination for the other food testing circumstances covered by the LAAF regulation. We will publish one or more additional notices in the Federal Register when the LAAF program attains sufficient laboratory capacity to support the food testing described in § 1.1107(a)(1)-(3).
Program Application
View LAAF Participant Information
- Recognized Accreditation Bodies
- LAAF-Accredited Labs
- LAAF-Accredited Laboratory Scopes
Create accounts and upload documentation for Recognized Accreditation bodies and LAAF-Accredited Labs.
- Application - Apply to become an Accreditation Body. Select "Create New Account" and then select the appropriate box under FSMA.
- Accreditation Bodies User Guide
- Accredited Laboratories User Guide
Final Rule
The FDA Food Safety Modernization Act (FSMA) final rule on Laboratory Accreditation for Analyses of Foods (LAAF) establishes a laboratory accreditation program for the testing of food in certain circumstances. Under the LAAF program, FDA will recognize accreditation bodies (ABs) that will accredit laboratories to the standards established in the final rule (referred to as LAAF-accredited laboratories).
The final rule specifies eligibility requirements that ABs and laboratories wishing to participate in the program will need to satisfy, as well as procedures for how the FDA will manage and oversee the program. In certain circumstances, owners and consignees will be required to use a LAAF-accredited laboratory for food testing. FDA will maintain an online public registry listing recognized accredited bodies and LAAF-accredited laboratories.
The establishment of the LAAF program is intended to improve the accuracy and reliability of certain food testing through the uniformity of standards and enhanced FDA oversight of participating laboratories.
For additional information on the final rule, see
Resources
Who is affected by this final rule?
The LAAF final rule applies to accreditation bodies and food testing laboratories that wish to participate in the program. Their participation is entirely voluntary. In certain circumstances owners and consignees will be required to use LAAF-accredited laboratories to conduct food testing.
Does the LAAF final rule apply to all food testing?
No. Food testing, including environmental testing, is only required to be conducted by a LAAF-accredited laboratory under certain circumstances specified in the rule. For the purposes of this rule, “food” has the same definition as in section 201(f) of the Federal Food, Drug, and Cosmetic Act. It includes articles used for food or drink for man or other animals except that food does not include pesticides (as defined in 7 U.S.C. 136(u)).
What testing is covered under the LAAF final rule?
After the LAAF final rule is fully implemented, owners and consignees will be required to use a LAAF-accredited laboratory for food testing:
- to support removal from an import alert through successful consecutive testing (e.g., to get a food product or firm removed from the red list);
- to support admission of an imported food (e.g., articles of human or animal food, and U.S. goods returned that are articles of food) detained at the border because it is or appears to be in violation of the Federal Food, Drug, and Cosmetic Act (e.g., products that contain or appear to contain unapproved food additives, including unauthorized food contact substances);
- required by existing FDA food safety regulations, when applied to address an identified or suspected food safety problem (i.e., certain tests related to shell eggs, sprouts, and bottled drinking water);
- required by a directed food laboratory order, a new procedure being implemented in this final rule that will allow FDA to require use of a LAAF-accredited laboratory to address an identified or suspected food safety problem in certain, rare circumstances; and
- conducted in connection with certain administrative processes (e.g., testing submitted in connection with an appeal of an administrative detention order).
What is the LAAF program implementation timeline?
FDA is taking a stepwise approach to implementation of the LAAF program. On July 12, 2022, the FDA posted a registry of six FDA-recognized accreditation bodies for the LAAF Program. Laboratories may now apply to the recognized accreditation bodies for LAAF-accreditation. When there is sufficient LAAF-accredited laboratory capacity for the food testing covered by the final rule, the agency will publish a document in the Federal Register giving owners and consignees 6 months’ notice that they will be required to use a LAAF-accredited laboratory for such food testing. The agency may issue more than one Federal Register document as LAAF-accredited laboratory capacity is attained for various types of food testing described in the final rule.
See Frequently Asked Questions on FSMA for additional information.
Contact Us
For Questions about the LAAF Program Requirements and Eligibility, contact: FSMA Technical Assistance Network (TAN)
For Questions about the LAAF Program Application and Implementation, contact: FDALAAFInquiry@fda.hhs.gov
Voluntary Graphic Element & QR Code
A LAAF program QR code is available to assist FDA and stakeholders with communicating the status of participants in the LAAF program.
LAAF graphic elements are available to help increase awareness of the LAAF program.
Download these materials and read these guidelines for using them by visiting Voluntary Graphic Element & QR Code Guidelines.