Pharmaceutical Quality - Chemistry, Manufacturing & Controls (PQ/CMC)
On May 1, 2023, FDA published a federal register notice including a document of new PQ/CMC data elements and terminologies for public comment. FDA is only seeking comment on Chapter 2 of the associated document. Comments on Chapter 1 will not be addressed as that content was published for comment previously in March 2022. Click to view the notice and associated document. You may also download the new document directly from fda.gov by clicking this link.
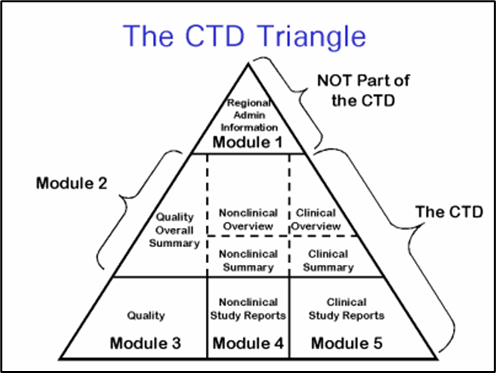
As part of an effort intended to support future electronic acquisition and use of submitted information, FDA has undertaken a project to identify and prioritize pharmaceutical quality/chemistry, manufacturing and controls (PQ/CMC) information that would benefit from a structured submission approach. This structured and standardized information is intended to be submitted in the Module 3 of the Common Technical Document as defined by the International Council for Harmonisation’s (ICH) M4 Common Technical Document (CTD). The effort is scoped to some of what is currently submitted in Module 3 and Module 2.3 of the electronic Common Technical Document (eCTD) submission. It is not intended to be comprehensive in structuring all eCTD product quality information, only those concepts that were considered amenable to structuring and would bring value to the quality review process.
The goal of this project is to establish electronic standards for submitting Pharmaceutical Quality (PQ) and Chemistry & Manufacturing Controls (CMC) data.
Specific objectives of the PQ/CMC Project are:
- Develop structured data standards for PQ/CMC
- Develop a data exchange standard for submitting the structured PQ/CMC data to the FDA
The submission of structured data in a standardized format should increase the efficiency of FDA’s review of PQ/CMC data contained in the Module 3 of eCTD submissions for a New Drug Application (NDA), an Investigational New Drug Application (IND), a Biologics License Application (BLA), an Abbreviated New Drug Application (ANDA), a New Animal Drug Application (NADA), an Abbreviated New Animal Drug Application (ANADA), an Investigational New Animal Drug (INAD), Generic Investigational New Animal Drugs (JINADs), and a Master File (MF).
For consistency of product quality data across FDA centers, the draft standardized data elements and terminologies were created by an Agency workgroup comprised of Subject Matter Experts (SMEs) from the Center for Drug Evaluation and Research (CDER), the Center for Veterinary Medicine (CVM), and the Center for Biologics Evaluation and Research (CBER).
The PQ/CMC Data exchange standard is intended to be developed as Health Level 7 (HL7) Fast Health Interoperable Resources (FHIR) representation. The project has been established with the HL7 Biomedical Research & Regulation (BR&R) work group and discussions regarding representing the PQ/CMC structured elements in FHIR occur during the work group meetings. These HL7 BR&R WG meetings are open to the public.
Figure 1: ICH CTD Modules
Stay Connected
- For additional information/support from PQ/CMC Team, please contact [email protected].